Proposed Implementation

Potential Detoxification Enzyme for Testing
We performed a literature review on enzymes that could be added to the engineered Snodgrasella alvi secretion system construct. A detoxification gene linked to the secretion tag of our construct would theoretically allow the enzyme to be produced and transported out of the cell into the bee gut, which is the principal location of bee insecticide metabolism [1].
We started our review with how insecticide metabolism is accomplished in insects. There are three phases of insecticide metabolism in all insects. Phase 1 is functionalization, in which enzymes chemically alter the insecticide so it can no longer bind to its target. This is done by cytochrome P450s and carboxylesterase enzymes. Phase 2 is conjugation, in which enzymes detoxify the Phase 1 products through conjugation to form water-soluble compounds that can then be eliminated . The enzymes that accomplish this are glutathione S- transferases and UDP-glycosyltransferases. The last phase is transport, which involves ABC transporters pumping the phase 2 conjugants out of the cell [2]. In many different insect species, an upregulation of these different enzymes provides enhanced resistance to pesticides [2,3,4,5].
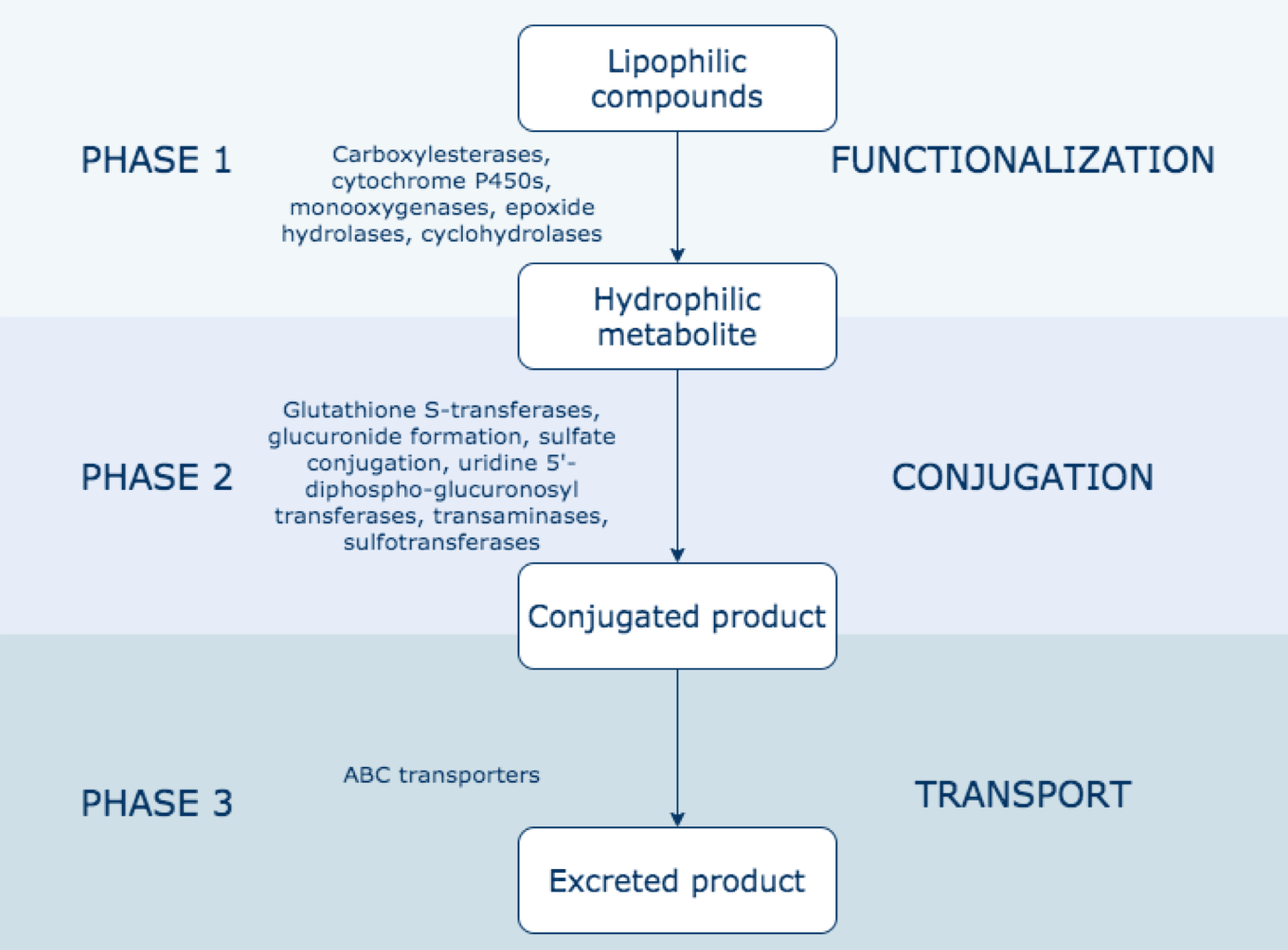
The different phases of insect metabolism. Figure from [3]
Interestingly, we found that bees have a deficit of each class of metabolism genes compared to other insects [6]. In fact, it has been found that bees upregulate their detoxification genes when exposed to the neonicotinoid pesticide, imidacloprid, which in turn causes a downregulation of genes for glycolysis and development [6]. This could explain why bees are so negatively impacted by pesticides. We decided the focus of our project would be to try to increase the amount of detoxification enzymes available in the bee gut. This would hopefully decrease the negative effects of pesticides on bees, as they wouldn’t have to downregulate important survival genes in order to upregulate detoxifying ones.
We propose that Glutathione S-Transferases (GSTs) enzymes should be implemented into the Bee-tox system in the future. GSTs work to detoxify xenobiotics such as pesticides by conjugation with glutathione. The active site residue of the GST interacts with GSH sulfhydryl group (-SH) to generate the catalytically active thiolate anion (GS-). This nucleophile then attacks the electrophilic center of any xenobiotic to form the corresponding GS-conjugate [7]. The resulting conjugated product is neutralized and water-soluble and can be excreted from the cell. GSTs also protect cells from oxidative damage. GSTs are very broad-acting enzymes and detoxify a wide range of electrophilic xenobiotics, since they utilize this same mechanism. We realized this was especially important after meeting with Dr. Milbraith, coordinator of the Michigan Pollinator Initiative. She informed us that commercial honeybees are exposed to a wide range of pesticides and fungicides, and that the combined effects of these toxins is incredibly harmful to bees. GSTs would be a great way to combat this issue, as they are all-encompassing when it comes to conjugating xenobiotics. In addition, previous studies have shown that recombinant bacterial and insect GSTs have been successful in detoxifying pesticides.
We decided to use GSTs from insects, as they will be the most similar to the GSTs found in bees and would most likely be more successful in detoxifying toxins in the bee midgut. In our literature review, we reviewed many different insect GSTs, but ultimately identified an Epsilon insect GST that we believe would be the best candidate for inserting into the Bee-tox system. This is HAGST-8, which originates in the insect Helicoverpa armigera. This particular gene was found when researchers from the Institute of Bioinformatics and Biotechnology fed Helicoverpa armigera larvae an insecticide mixture containing chlorpyrifos, dichlorvos and cypermethrin as part of their diet. They then amplified the HAGST-7 gene, and found a variant that differed in amino acid sequence from control larvae, so they named it HAGST-8 [8]. The results from their study showed us that this was a very promising candidate for the Bee-tox probiotic, and we will further explain this study’s findings and its significance.
The researchers cloned HAGST-8 into P. pastoris for extracellular secretion by inserting it into a secretion plasmid. The broth of the P. pastoris with the recombinant plasmid had 2x higher GST activity than the control without the plasmid. This demonstrates that a recombinant HAGST-8 enzyme was able to be produced, and had high activity when it was secreted. This is important for Bee-tox, as we worked on engineering a Type 1 secretion system in our chassis, S. alvi, to secrete a detoxficiation enzyme into the bee gut. Few recombinant insect GSTs have been tested for secretion, so this makes HAGST-8 an extremely promising candidate.
The researchers of this study then grew the recombinant HAGST-8 P. pastoris in different combinations of methanol (since expression of HAGST-8 was driven by an alcohol oxidase promoter that is induced by methanol) and a pesticide mixture. The results are shown below.

The P. pastoris that were not grown in methanol, and subsequently did not produce HAGST-8, had severely inhibited growth. The P. pastoris that did produce HAGST-8 were able to grow in the presence of the pesticide mixture, and greater growth was actually observed in these yeast than in the ones grown without pesticides. This demonstrates that the recombinant HAGST-8 conferred resistance to the P. pastoris against the pesticide mixture. This showed us that HAGST-8 could potentially substantially reduce the damage inflicted by pesticides in bees if it was secreted into their gut.
The purified HAGST-8 was then purified and incubated in a mixture of pesticides. This was done alongside a crude GST enzyme extract from H. ameriga and empty vector proteins. The percent of pesticide reduction was then monitored by GC-MS.

The empty vector proteins only reduced the pesticides by 0-10%, which eliminates the possibility of the pesticides binding to non-specific proteins. The recombinant HAGST-8 had a similar percent reduction in pesticides as the crude enzyme extract, proving the recombinant GST is effective at detoxifying pesticides. The pesticides were then treated with the purified HAGST-8 for 4 hours and analyzed by LC-HRMS, there was a complete reduction of chlorpyrifos and dichlorvos, and a 53% reduction of cypermethrin. This demonstrates that the recombinant HAGST-8 is extremely effective at detoxifying these pesticides.
Lastly, the researchers of this study generated the 3D structure of HAGST-7 and HAGST-8 and used PatchDock to analyze the molecular docking with different pesticides. HAGST-8 had a significantly higher binding affinity to neonicotinoid pesticides, such as imidacloprid and thiacloprid than HAGST-7. The differential binding affinities between the two enzymes could be because HAGST-8 originated in larvae exposed to pesticides. The figure below shows a heatmap of the binding strength of both HAGST-7 and HAGST-8 .


From all of the insect GSTs we reviewed in our literature search, HAGST-8 seems the most promising to have the greatest impact on bees. First, HAGST-8 was successfully cloned into yeast and secreted. The secreted HAGST-8 was active, and conferred resistance to the yeast exposed to pesticides. This is very important as the enzyme inserted into our project would also be excreted from S. alvi and need to function extracellularly in the bee gut. Also, HAGST-8 was very effective at conjugating mixtures of pesticides, which is what we would want a detoxification enzyme in our probiotic to do. Lastly, HAGST-8 showed a much higher binding affinity to neonicotinoid pesticides, which is the focus of our detoxification efforts in bees. Little research has been done on GST conjugation of neonicotinoids, so this is a promising sign.
We propose that in the future, HAGST-8 should be amplified and cloned into the engineered pBTK510 plasmid we created carrying the Type 1 secretion system genes and secretion tag. This plasmid should then be conjugated into S. alvi, the natural bee gut microbe acting as our chassis organism. HAGST-8 was cloned into yeast in this study, so it will be important to see if it is able to be produced and secreted in our bacterial chassis. The supernatant, which should contain the secreted HAGST-8 should then be analyzed with a GST colorimetric assay using CDNB as a substrate. GST activity is determined by measuring the rate of conjugation of CDNB with glutathione, which is proportional to the increase in absorbance at 340 nm over time. Plotting the absorbance value OD340nm over a 4 minute interval, collecting data every 0.5 minutes, would allow future researchers to calculate the GST activity in GST units. If this experiment was successful, and the GST activity was comparable to the one the researchers of the above study found in the broth of the P. pastoris (1643.53 Units), the HAGST-8 transformed S. alvi could then be fed to newly emerged honeybees in the laboratory. The honeybees with the probiotic would then be fed a pesticide, such as imidacloprid, in their diet. It could then be observed if the bees with the S. alvi producing HAGST-8 have a higher survival rate. This would provide data on whether the Bee-tox probiotic is successful at detoxifying pesticides in the bee gut and confers resistance to bees. We hope that this proposed implementation of HAGST-8 into the Bee-tox secretion plasmid, and the subsequent experiments to determine its efficacy, can be tested sometime soon in the future.
Potential Bee-tox Biocontainment Mechanism
The overall goal for Bee-tox is that one day it will be administered to bees in the real world. One important consideration for any synthetic biology project is biocontainment, which is the prevention of the release of the recombinant genes into the environment, where they can no longer be controlled. This is especially important for our project, as Bee-tox was designed with the goal of being administered to either wild bees or commercial honeybees that farmers rely on to pollinate their crops. Bees have high exposure to pesticides as farmers spray them on their fields to protect their crops. So, it is imperative that a detoxification gene is not integrated into the microbiota of pests, allowing them to be resistant to the pesticide too!
The first line of defense that will prevent the recombinant genes in our probiotic from spreading to other organisms is the fact that S. alvi is only known to survive in the bee gut, so the bacteria itself will most likely not be able to establish in any other insects. Also, S. alvi has very specific growing conditions, such as a 5% CO2, meaning it extremely unlikely any excreted S. alvi from the bee would be able to survive in atmospheric conditions for long [1].
We researched various biocontainment mechanisms that could be engineered into our chassis to prevent our plasmid from being transferred to other bacteria in the environment, either through horizontal gene transfer or a phage transfer. The genetic biocontainment mechanism we propose for future implementations of our probiotic combines two genetic systems. The first is to utilize a T7 polymerase, which is a highly specific polymerase that is found in the T7 bacteriophage that infects E. coli [2,3,4]. The T7 polymerase will only recognize and transcribe genes that have a T7 promoter at the beginning of them [2]. Without this polymerase, genes under the control of a T7 promoter cannot be transcribed. The T7 system is often used to increase expression of recombinant proteins in bacteria, as T7 polymerase is very active and allows for high expression [3]. Our plan is to integrate the T7 polymerase gene into the S. alvi genome using CRISPR, under the control of a constitutive promoter. We would then attach the T7 promoter to the detoxification gene that is located on our vector. Only the T7 polymerase would be able to transcribe this gene, which the S. alvi would synthesize from the gene inserted into its chromosome. If the plasmid was transferred to another bacterium, the detoxification gene would be rendered useless without the T7 polymerase initiating transcription at the T7 promoter. The T7 polymerase only occurs naturally in the T7 bacteriophage, and has not been found in any naturally occurring microbe. The T7 promoter is also long enough that it’s unlikely to occur by chance in any DNA unrelated to T7 DNA [1]. Furthermore, the T7 expression system has been tested and expressed before in S. alvi [5].Even though it was found to have a low expression of GFP under a T7 promoter, this actually could make it more feasible in the long-term as oftentimes the T7 Polymerase is very active and can overwhelm cells’ metabolism [2]. The detoxification gene would be more likely to stably exist in S. alvi if the T7 polymerase is less active.
In addition to utilizing the T7 Polymerase and promoter system, we also plan to integrate the GhoST toxin-antitoxin system as another layer of biocontainment to prevent the uptake of this gene by other bacteria. This system was used by MSU’s 2019 iGEM team. GhoT is a helical protein toxin that disrupts the cell membrane and leads to the formation of ghost cells (dying cells with a damaged membrane) [5]. GhoT works by reducing the intracellular ATP and disrupting the cell membrane potential [6]. GhoS is the anti-toxin protein that cleaves the mRNA of GhoT and prevents its production. GhoS and GhoT genes form an operon and are expressed together, although studies have introduced one gene or the other into cells [5]. So, when GhoS and GhoT are both expressed, the cell is able to survive. When only GhoT is expressed, the cell will either die or go into a dormant state.
We propose to integrate the GhoS antitoxin onto the S. alvi chromosome and add the GhoT toxin to our detoxifying plasmid. This way, only the recombinant S. alvi will have both proteins needed to survive. If the plasmid is transferred to another bacteria via horizontal gene transfer, the GhoT gene present on the plasmid will kill the new cell. We believe this system has a lot of potential in our probiotic, as GhoS expression is not inhibited by stress, as is the case for many other anti-toxins [6].
In summary, a pBTK510 plasmid, which was the most successful vector in transforming S. alvi in the Bee Microbiome Toolkit [4], will contain the detoxification gene, under the control of the T7 promoter and terminator. The plasmid will also contain the GhoT toxin gene. Once that plasmid has been conjugated into S. alvi, we plan to use CRISPR to introduce two genes onto the S. alvi chromosome: GhoS and T7 polymerase.

This shows the genetic engineering plan for biocontainment.
We believe that our biocontainment plan would be very secure and effectively prevent gene transfer of the detoxification gene into other environmental bacteria. The event that all of these genes, located on a plasmid and on two separate locations on the chromosome, would all be transferred to another bacteria is unlikely.
The plan to test this biocontainment mechanism would be to add the T7 system in S. alvi first. Once the T7 polymerase gene is confirmed to have been integrated into the chromosome, we would conjugate the S. alvi with the plasmid containing the detoxification gene with the T7 promoter. We would then collect data on the activity level of the detoxification enzyme being produced, using an assay for the detoxification enzyme. If there is a significant level of enzyme activity, we can then combine our systems by integrating all of the parts into S. alvi, as shown above in Figure 1 to test if the S. alvi is able to survive and replicate, as well as determine the enzyme activity level being produced. Hopefully, we can one day test our ideas to assess the feasibility of this project being implemented in commercial honey bees or wild bees out in the field.
Bee-tox administration via Feeding Device
We have considered several avenues through which our engineered probiotic may be administered to both wild and commercial bee populations.
Commercial/Hobby Honey Bees
Due to the insular nature of honey bee colonies, administration of engineered S. alvi to commercial hives would be relatively straightforward. We discussed current methods of probiotic administration to commercial and hobby bee colonies with Brian Peterson and Dr. Megan Millbrath, who pointed us towards several options. We initially proposed a simple feeding device filled with a sucrose-water solution containing the probiotic that could be inserted into a colony. However, the experts we spoke to recommended a method commonly used to administer treatments to hives, which is to freeze-dry the engineered probiotic and mix with powdered sugar. The mixture may then be spread on the frames of the hive.
Because members of the bee gut microbiome are transmitted socially in bees living in colonies, we hope that the inoculation of even a few bees within a hive with the engineered probiotic would be sufficient in order to establish it throughout the colony.
Wild Bees
Although honey bees kept for commercial use are at the highest risk of repeatedly encountering crops treated with pesticides, wild bees are also endangered by neonicotinoid usage, even at sublethal doses [1]. However, probiotic administration to wild bee populations poses several unique challenges. First, due to the solitary nature of many wild bee species, transmission of the probiotic through social contact or by implementing feeders within a hive is unlikely to be effective. Biocontainment is also a concern with the proposed implementation of our product, as spread of the probiotic to non-target insects such as crop-threatening pests may have a negative impact on the agricultural industry. For these reasons, we decided to design a prototype for a wild bee feeder designed to attract wild bees and restrict probiotic access for non-target organisms. The feeder is equipped with an image-recognition system designed to allow access to the feeding solution only when a bee has been detected. Images of the feeder can be found on the design page under the Device section and a downloadable file for the 3D printed portion can be found on the contribution page.

The prototype is designed for placement level with the herb layer (layer of vegetation where bees can be observed foraging).
We propose a 1:1 sucrose:water solution containing the engineered S. alvi and antibiotic (to which the engineered microbe will be resistant) to prevent contamination. Further experimentation is necessary to determine engineered S. alvi viability in sugar solution in order to provide detailed instructions for feeder replenishment. Further experimentation is also necessary to determine effectiveness of potential attractants such as lemongrass oil.
References
Potential Detoxification Enzyme
- Severine Suchail, et al. “In vivo distribution and metabolisation of 14C-imidacloprid in different compartments of Apis mellifera.” Pest Management Science, vol. 60, 2004, pp.1056–1062., doi:10.1002/ps.895
- Højland DH, Kristensen M (2017) “Analysis of Differentially Expressed Genes Related to Resistance in Spinosad- and Neonicotinoid- Resistant Musca domestica L. (Diptera: Muscidae) Strains.” PLoS ONE 12(1): e0170935. doi:10.1371/ journal.pone.0170935
- Chen, Xiaokun, et al. “The Cross-Resistance Patterns and Biochemical Characteristics of an Imidacloprid-Resistant Strain of the Cotton Aphid.” Journal of Pesticide Science, vol. 40, no. 2, 8 Jan. 2015, pp. 55–59., doi:10.1584/jpestics.d14-031.
- Reid, William R, et al. “Overexpression of a Glutathione S-Transferase (Mdgst) and a Galactosyltransferase-like Gene (Mdgt1) Is Responsible for Imidacloprid Resistance in House Flies.” Pest Management Science, vol. 75, no. 1, 2018, pp. 37–44., doi:10.1002/ps.5125.
- Wei, Zi-Han. “ Overexpression of Glutathione S-Transferase Genes in Field λ-Cyhalothrin-Resistant Population of Cydia pomonella : Reference Gene Selection and Expression Analysis.” Journal of agricultural and food chemistry, vol. 68, no. 21, May 2020, pp. 5825-5834., doi: 10.1021/acs.jafc.0c01367
- Berenbaum, May, R, et al. “Xenobiotic detoxification pathways in honey bees.” Insect Science, vol. 10, August 2015, pp. 51-58., doi:10.1016/j.cois.2015.03.005
- Richard N. Armstrong. “Structure, Catalytic Mechanism, and Evolution of the Glutathione Transferases.” Chemical Research in Toxicology, 1997 10 (1), 2-18. doi: 10.1021/tx960072x
- Labade, Chaitali, P, et al. “Role of induced glutathione-S-transferase from Helicoverpa armigera (Lepidoptera: Noctuidae) HaGST-8 in detoxification of pesticides” Ecotoxicology and Environmental Safety, vol 147, January 2018, pp. 612-62., doi:10.1016/j.ecoenv.2017.09.028
Potential Bee-tox Biocontainment Mechanism
- Kwong, W, and Moran, N. “Cultivation and characterization of the gut symbionts of honey bees and bumble bees: description of Snodgrassella alvi gen. nov., sp. nov., a member of the family Neisseriaceae of the Betaproteobacteria, and Gilliamella apicola gen. nov., sp. nov., a member of Orbaceae fam. nov., Orbales ord. nov., a sister taxon to the order ‘Enterobacteriales’ of the Gammaproteobacteria.” International Journal of Systematic and Evolutionary Microbiology, vol. 63, no. 6, 2013, pp.2008-2018., doi: 10.1099/ijs.0.044875-0
- Wycuff, D and Matthews, K. “Generation of an AraC-araBAD Promoter-Regulated T7 Expression System.” Analytical Biochemistry., vol. 277, 2000, pp. 67-73., doi:10.1006/abio.1999.4385 Studier, W and Moffattf , B. “Use of Bacteriophage T7 RNA Polymerase to Direct Selective High-level Expression of Cloned Genes.” J. MoZ. Biol, vol 189, 1986, pp. 113-130., doi:10.1016/0022-2836(86)90385-2
- Kang, Y, et al. “One step engineering of T7-expression strains for protein production: Increasing the host-range of the T7-expression system.” Protein Expression and Purification, vol. 55, no. 2, Oct. 2007, pp. 325-333., doi:/10.1016/j.pep.2007.06.014
- Leonard, S et. al. “Genetic Engineering of Bee Gut Microbiome Bacteria with a Toolkit for Modular Assembly of Broad-Host-Range Plasmids.” ACS Synth Biol., vol. 7, no. 5, May 2018, pp. 1279-1290., doi: 10.1021/acssynbio.7b00399 Wang, X, et al. “A new type V toxin-antitoxin system where mRNA for toxin GhoT is cleaved by antitoxin GhoS.” Nature Chemical Biology, vol. 8, no.10, Sept. 2012, pp.855-861., doi: 10.1038/nchembio.1062
- Cheng, H, et. al. “Toxin GhoT of the GhoT/GhoS toxin/antitoxin system damages the cell membrane to reduce adenosine triphosphate and to reduce growth under stress.” Environmental Microbiology, vol. 16, no.6, Dec. 2013, pp. 1741-1754., doi:10.1111/1462-2920.12373
- Kwong, W., Moran, N. Gut microbial communities of social bees. Nat Rev Microbiol 14, 374–384 (2016). https://doi.org/10.1038/nrmicro.2016.43
Bee-tox administration via Feeding Device
- Rundlöf, M., Andersson, G., Bommarco, R. et al. Seed coating with a neonicotinoid insecticide negatively affects wild bees. Nature 521, 77–80 (2015). https://doi.org/10.1038/nature14420

