Project Title:
Creation of a 3D-bioprinted polycaprolactone scaffold with mussel-foot protein Pvfp-5β-based bioadhesive coating for biomedical applications.
Project Abstract:
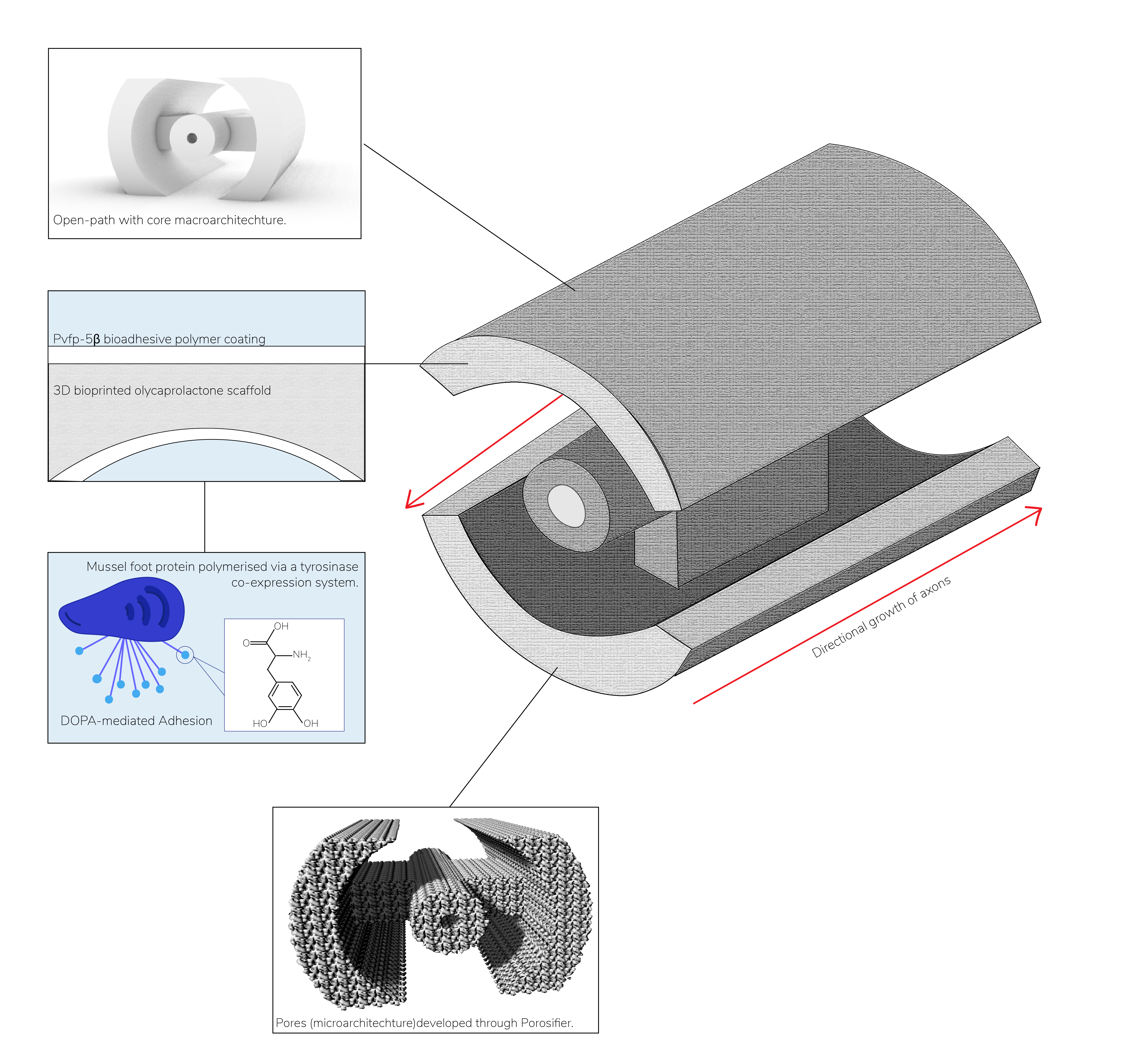
Annually, between 250,000 and 500,000 individuals worldwide suffer from a spinal cord injury (SCI). SCI is characterised by damage to the spinal cord followed by a complex pathophysiological response and loss of neuronal function below the site of injury. The limited regenerative abilities of the CNS combined with the inhibitory environment created by the glial scar at the affected area pose numerous challenges to restoring function. Working towards a therapy for SCI, we have designed and modelled a biodegradable scaffold composed of polycaprolactone, that incorporates a synthetic mussel-foot protein based bioadhesive coating, to encourage axonal attachment, and can be produced using 3D-bioprinting methods. Our scaffold is customisable and contains the necessary micro- and macro-architectures predicted to topographically encourage axonal regrowth and withstand the mechanical forces in the spine. We have further investigated in silico the physicochemical properties of our chosen protein Pvfp-5β to better understand its biotherapeutic use.
Authors:
All authors are based at King's College London (London, United Kingdom) and are listed in alphabetical order.
Abigail Conner, Alya Masoud Abdelhafid, Ela Kanani, Emily Blundell, Gonzalo Leon Gonzalez, Harshraj Bumia, Ilaria Franceson, Jasmin Werner, Kyriakos Attouni, Kurt Peng, Leon Yong Kang Zhang, Liyamu Ma, Luke Bateman, Morgan Zvezdov, Peter Kanyike, Remy Tran, Shams Almusawi, Sonya Panchenko, and Stephanie Avraamides.
Sponsors:
We would like to express our sincerest thanks to Promega for their generous sponsorship. Additionally, we would like to thank King's College London for further funding. Thank you to SnapGene, Geneious, BMG Labtech, and The Biochemical Society for providing us with the software and financial assistance that has underpinned this project. We would also like to express our thanks to those who donated for our GoFundMe campaign.