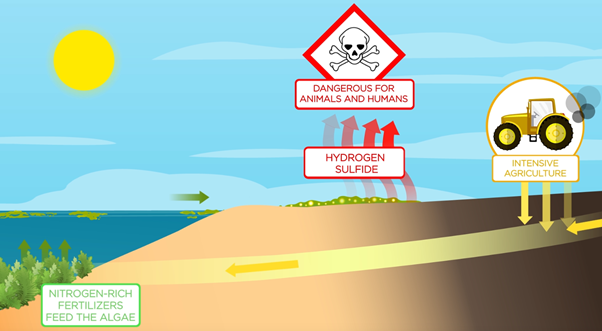
Nitrogen comes naturally from the degradation of organic matter. But fertilizers used in intensive agriculture to feed the plants, go into the sea and enrich the nitrogen composition [1]. Under normal circumstances, nitrogen therefore appears to be the main limiting element of the growth of green algae. It defines the nitrophilic character of these plants. According to the Regional Directorate for the Environment, Planning and Housing of Brittany (DREAL Bretagne), it has been more than 40 years since nitrate levels in water of surface have exceeded the threshold of 10 mg /L below which the green algae would no longer be saturated with nitrogen. [2] [3].
The green algae on the beach decompose and produce, between other gases, hydrogen sulfide, a deadly gas for animals and humans. Indeed, the elements essential for the proper growth of ulva are mainly carbon dioxide (COO2), nitrogen (N), phosphorus (P), then in smaller quantities, silica and iron. It is therefore a major health risk. Because of olfactory and visual nuisances, green tides also have a strong impact on tourist numbers. It is therefore imperative to collect this large quantity of green algae which must be destroyed or recovered.
The retrieval of those algaes is done through mechanical and human means depending on their quantity and accessibility, however both of those means are not without their flaws: mechanical gathering take a lot of sand away from the beaches, accentuating their erosion problems while cleaning through human manpower is pretty slow in comparison and require an investment in extensive (and often expensive) protection measures as the workers work in a toxic environment [1]. It is also good to note that another method exists but is also far from perfect: the gathering of algaes in the sea curtain of the bottom beach. There the algaes are trapped before they reach the shore and, as they are still underwater, they survive until they are claimed. In that state the seaweeds can’t degrade and produce hydrogen sulfide and are still fresh when retrieved which allow for a far wider range of possibilities to be reused. Even better, this method also works outside of green tides periods, reducing the quantities of algaes outside of their growing periods. However the problem is that this technique has a negative impact on the bottom beach fauna [4].
According to data from the Launay-Lantic Organic Valorization Unit (UVO), they collected 3.230 tons of algae in 2015 [4].
The management of stranded biomass has also become a major issue in itself. Each year, collecting seaweed is very expensive for many local communities. For example, in Brittany (France) in 2009, the gathering of the macroalgaes costed 888 000 € for 68 928 t [5]. If we add treatment cost and the retrieval in the sea curtain (for the year 2010) the cost jumped to 1 559 000 €. Various recycling possibilities have therefore been considered, in order to reduce costs, and even to recover, these thousands of tons of washed up seaweed. While these are still very little exploited at present, many studies have demonstrated the multiple possibilities of valuing green algae in the food, agricultural and health industries, but also in the energy field.

Picture 1. Green algaes stranded on a beach
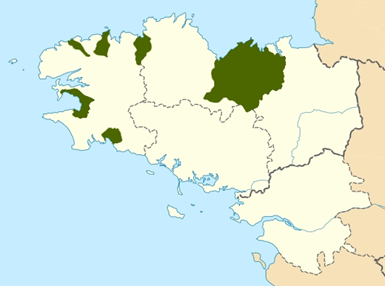
Fig 2. In green are the more nitrogen contaminated watersheds (map of Brittany, France)
In the midst of these green tides, we mainly find Ulves species (Ulva rotundata bay of St-Brieuc and Ulva armoricana harbor of Brest). We therefore decided to work on these green algae species as a substrate to develop our project. Due to its high photosynthesis capability, they have sufficient carbon for usage in biorefinery.
Ethanol production from algae is based on fermentation of algal polysaccharides which are starch, sugar and cellulose. Another polysaccharide from macroalgae is ulvan. It is mainly presented in Ulva sp. and it is a source of sugars for production of fine chemicals. This gelling sulfated polysaccharide is composed of xylose, glucose, galactose but mainly on rhamnose ! [6]

Picture 2. Ulva armoricana
Rha
Glc
Xyl
GlcA
IduA
CH total
Degree sulfation
32.0
7.5
5.8
20.8
12.1
78.3
8.7± 0.5
Rha=Rhamnose, Gal = Galactose, Glc = Glucose, Xyl = Xylose, GlcA = Glucoronic acid, and IduA = Iduronic acid 3.
Several studies have already made it possible to create optimized biological systems for the production of ethanol in biofuels from glucose and xylose as a substrate, but few studies have looked at production from rhamnose.
One very promising route for valorization is the production of bioethanol that could be blended with gasoline and used by adapted vehicles. This would make it possible to reduce the use of fossil fuels while representing a source of income for the impacted regions.
Negative environmental consequences of fossil fuels and concerns about petroleum supplies have spurred the search for renewable transportation biofuels. To be a viable alternative, a biofuel should provide a net energy gain, have environmental benefits, be economically competitive, and be producible in large quantities without reducing food supplies.
Bioethanol, which is also known as ethyl alcohol or bioethanol fuel, is the most widely used liquid biofuel. It can be directly used as sole fuel in vehicles or partially blended with gasoline effectively. In 2018, the global production of bioethanol had reached 28.7 billion gallons. The current biggest producer of bioethanol in the world is the United States (USA) and followed by Brazil. Besides, it has also been integrated into the east countries’ market, with the majority in China, India, Philippines, Thailand, and Korea [7].
Bioethanol can also be categorized into first, second, and third generations according to the various feedstocks. The first-generation bioethanol is produced from food crops such as barley, wheat, corn, and sugar cane. Although the production technology for first-generation bioethanol has been well-established, the demand for food crops in production inflates food prices and exacerbates the food scarcity problem. Hence, the focus has shifted to the second-generation ethanol that is derived from non-edible lignocellulosic biomass. Nevertheless, second-generation bioethanol faced technical issues due to the presence of lignin in the feedstock. The delignification process is technically demanding and cost-intensive, hence impedes the commercialization of second-generation bioethanol. This drives the use of third-generation feedstock that are micro- or macroalgae. The production of bioethanol from macroalgae is more promising as macroalgae do not require land and freshwater for cultivation as they have a fast growth rate, high carbohydrate content as well as low lignin levels simplifies the hydrolysis stages [7] [8].
Feedstock
Ethanol yield (gal/acre)
Ethanol yield (L/ha)
Corn stover
112-150
1,050 - 1,400
Wheat
277
2,590
Cassava
354
3,310
Sweat sorghum
326 - 435
3,050 - 4,070
Corn
370 - 430
3,460 - 4,020
Sugar beet
536 - 714
5,010 - 6,680
Switch grass
1,150
10,760
Algea
5,000 - 15,000
46,760 - 140,290
Our aim is to develop engineered microorganisms able to transform rhamnose in bioethanol from Ulvane. We envision that our engineered strains could be used in industrial large-scale fermenters in order to provide a valorization solution for green algae and make the production of ethanol profitable.
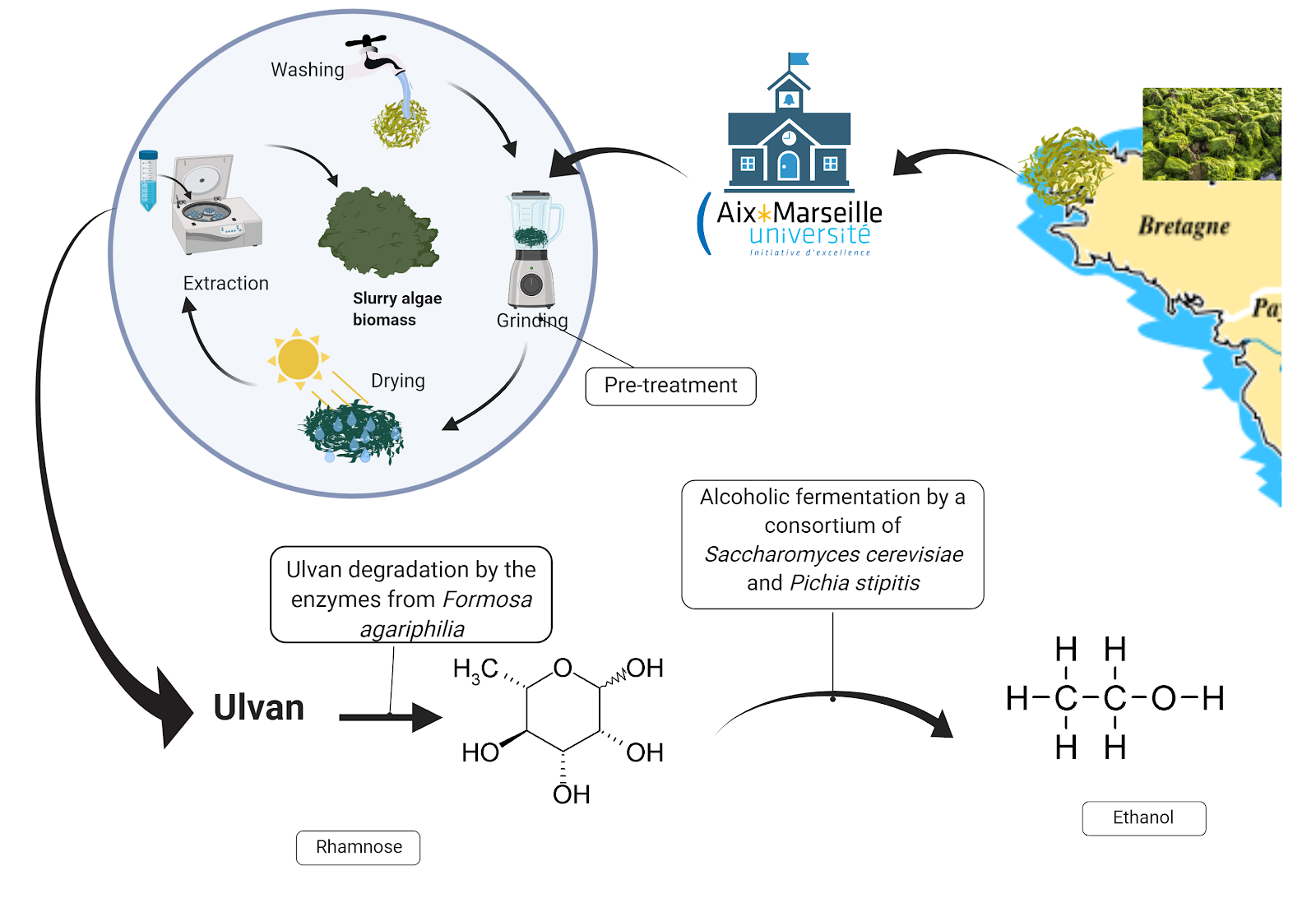
Fig 3. Schematic representation of the different steps needed to transform green algaes into ethanol @created by biorender
During the genesis of the project, we identified that the use of ulves as a resource for bioethanol production could be separated into two successive tasks: ulvan degradation/saccharification and alcoholic fermentation. In order to monitor rhamnose production, we also decided to include a rhamnose biosensor in the design.
For knowing the Rhamnose concentration and cooperate with our further tests, the final biomass would be tested with our biosensor system explained below.
- Ulvan degradation/saccharification: The next step consists of breaking down Ulvan into monosaccharide. During our research, we had the opportunity to discuss with the researcher Philipe Pottin who suggested an article that appeared recently about a bacteria, Formosa agariphila. This bacteria has been found living with algae and contains a PUL (polysaccharide utilization locus) with multiple enzymes that naturally depolymerize sugar polymers [9]. From the literature, we identified a minimum pathway of four enzymes from F. agariphila (two ulvan lyases P30-PL28; P10-PLnc, the unsaturated-3S-ramnoglycuronyl hydrolase P33-GH105 and a sulfatase P36-S1-25) that seems sufficient to break down ulvan into rhamnose and other sugars. We plan to have those gene sequences synthesized by Twist. We will first clone those sequences into bacterial expression vectors and introduce them into E. coli W3110 in order to test protein production and activity on polysaccharides. Using this chassis, we expect to validate a minimal pathway for rhamnose production from ulvans.
- Alcoholic fermentation : In a second step, we need to convert the rhamnose (and other sugars resulting from ulvan degradation) into bioethanol. Then, because yeast is a more attractive chassis than E. coli for large-scale production of proteins, we plan to introduce our four validated biobricks (codon optimized) into yeast expression vectors carrying a sequence for protein secretion. This will assure that the yeast Saccharomyces cerevisiae will be able to produce and to secrete the ulvan degradation enzymes, allowing rhamnose production in the culture medium. S.cerevisiae is able to quickly ferment some sugar like glucose and xylose but not rhamnose.
Therefore, we need to create a consortium with another yeast to help fermentation. Pichia stipitis has already been used with S.cerevisiae for saccharification step, and have shown a better ethanol production in consortium than yeast alone [10]. P.stipitis is able to use rhamnose and transform it into pyruvate. This substrate can be used by S.cerevisiae. - Rhamnose detection : In order to measure rhamnose production in the culture supernatant, we decided to take advantage of an existing biobrick from the registry (BBa_K914003): a rhamnose-responsive promoter from Paris Bettencourt team 2012. The promoter will be fused to RFP and further characterized as a rhamnose biosensor. This characterization will also rely on recent literature on the rhamnose operon [11].
Yeast
Initial sugar (gs/L)
Residual sugar (gs/L)
Sugar consumption (%)
Ethanol (gp/L)
Sugar conversion to ethanol (%)
S.cerevisiae
50
15
70
16.7
92
P.stipitis
50
19
62.2
13.3
82
S.cerevisiae + P.stipitis
50
6
88
21.6
96