| Line 555: | Line 555: | ||
subsequent wet lab troubleshooting harder, as well as change the dry lab | subsequent wet lab troubleshooting harder, as well as change the dry lab | ||
output in unreasonable ways, as the TEV protease would then have different | output in unreasonable ways, as the TEV protease would then have different | ||
| − | efficiencies depending on the used recognition site ( | + | efficiencies depending on the used recognition site (Dougherty et al., 1989). In order to limit the number of |
| − | + | unknowns, and | |
to have the same affinity of our TEV protease to our recognition site, | to have the same affinity of our TEV protease to our recognition site, | ||
we opted to use the same recognition site everywhere; <b>ENLYFQG.</b> | we opted to use the same recognition site everywhere; <b>ENLYFQG.</b> | ||
| Line 617: | Line 617: | ||
<a href="#receptor-ref" aria-label="Back to content">↩</a></li> | <a href="#receptor-ref" aria-label="Back to content">↩</a></li> | ||
<li id="organism">Gunde T, Barberis A. Yeast growth selection system for detecting activity and inhibition of dimerization-dependent receptor tyrosine kinase. Biotechniques. 2005;39(4):541-549. doi:10.2144/000112011<a href="#organism-ref" aria-label="Back to content">↩</a></li> | <li id="organism">Gunde T, Barberis A. Yeast growth selection system for detecting activity and inhibition of dimerization-dependent receptor tyrosine kinase. Biotechniques. 2005;39(4):541-549. doi:10.2144/000112011<a href="#organism-ref" aria-label="Back to content">↩</a></li> | ||
| − | <li id="organism"> | + | <li id="organism">Snider, J., Kittanakom, S., Curak, J., & Stagljar, I. (2010). Split-ubiquitin based membrane yeast two-hybrid (MYTH) system: A powerful tool for identifying protein-protein interactions. Journal of Visualized Experiments. https://doi.org/10.3791/1698<a href="#organism-ref" aria-label="Back to content">↩</a></li> |
<li id="vp16">Dossani, Z. Y., Reider Apel, A., Szmidt-Middleton, H., Hillson, N. J., Deutsch, S., Keasling, J. D., & Mukhopadhyay, A. (2018). A combinatorial approach to synthetic transcription factor-promoter combinations for yeast strain engineering. Yeast. https://doi.org/10.1002/yea.3292/<a href="#vp16-ref" aria-label="Back to content">↩</a></li> | <li id="vp16">Dossani, Z. Y., Reider Apel, A., Szmidt-Middleton, H., Hillson, N. J., Deutsch, S., Keasling, J. D., & Mukhopadhyay, A. (2018). A combinatorial approach to synthetic transcription factor-promoter combinations for yeast strain engineering. Yeast. https://doi.org/10.1002/yea.3292/<a href="#vp16-ref" aria-label="Back to content">↩</a></li> | ||
<li id="tev">Wehr, M. C., Laage, R., Bolz, U., Fischer, T. M., Grünewald, S., Scheek, S., Bach, A., Nave, K. A., & Rossner, M. J. (2006). Monitoring regulated protein-protein interactions using split TEV. Nature Methods. https://doi.org/10.1038/nmeth967<a href="#tev-ref" aria-label="Back to content">↩</a></li> | <li id="tev">Wehr, M. C., Laage, R., Bolz, U., Fischer, T. M., Grünewald, S., Scheek, S., Bach, A., Nave, K. A., & Rossner, M. J. (2006). Monitoring regulated protein-protein interactions using split TEV. Nature Methods. https://doi.org/10.1038/nmeth967<a href="#tev-ref" aria-label="Back to content">↩</a></li> | ||
| Line 626: | Line 626: | ||
<li id="site">Hladek, M. D., Szanton, S. L., Cho, Y. E., Lai, C., Sacko, C., Roberts, L., & Gill, J. (2018). Using sweat to measure cytokines in older adults compared to younger adults: A pilot study. Journal of Immunological Methods. https://doi.org/10.1016/j.jim.2017.11.003/<a href="#site-ref" aria-label="Back to content">↩</a></li> | <li id="site">Hladek, M. D., Szanton, S. L., Cho, Y. E., Lai, C., Sacko, C., Roberts, L., & Gill, J. (2018). Using sweat to measure cytokines in older adults compared to younger adults: A pilot study. Journal of Immunological Methods. https://doi.org/10.1016/j.jim.2017.11.003/<a href="#site-ref" aria-label="Back to content">↩</a></li> | ||
<li id="vp16">Peters, V. A., Joesting, J. J., & Freund, G. G. (2013). IL-1 receptor 2 (IL-1R2) and its role in immune regulation. In Brain, Behavior, and Immunity. https://doi.org/10.1016/j.bbi.2012.11.006/<a href="#vp16-ref" aria-label="Back to content">↩</a></li> | <li id="vp16">Peters, V. A., Joesting, J. J., & Freund, G. G. (2013). IL-1 receptor 2 (IL-1R2) and its role in immune regulation. In Brain, Behavior, and Immunity. https://doi.org/10.1016/j.bbi.2012.11.006/<a href="#vp16-ref" aria-label="Back to content">↩</a></li> | ||
| − | |||
</ol> | </ol> | ||
Revision as of 21:31, 25 October 2020

So... how do you want the yeast to sense interleukins?
How nice of you to ask! Yeast doesn’t have endogenous receptors for any interleukins,
as opposed to humans. Actually, Saccharomyces cerevisiae doesn’t have any
Receptor Tyrosine Kinase type receptors (RTKs) - a category that most interleukin receptors fall under . RTKs are systems where two or more receptors associate
and start an autophosphorylation reaction because of that, eventually leading to an intracellular signaling cascade. This, we quickly realized, would be hard to replicate in yeast.
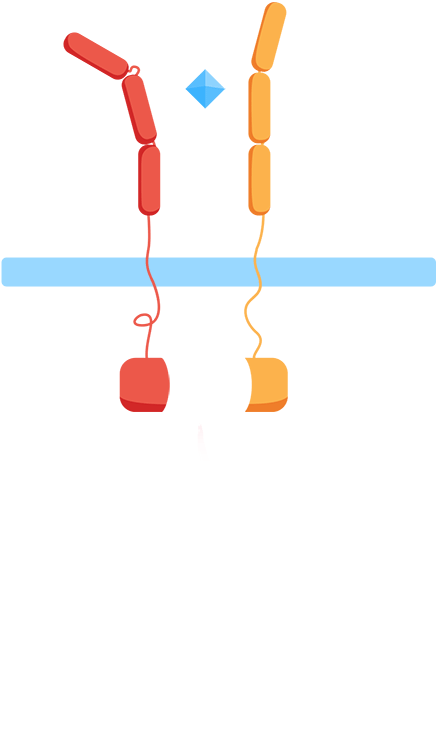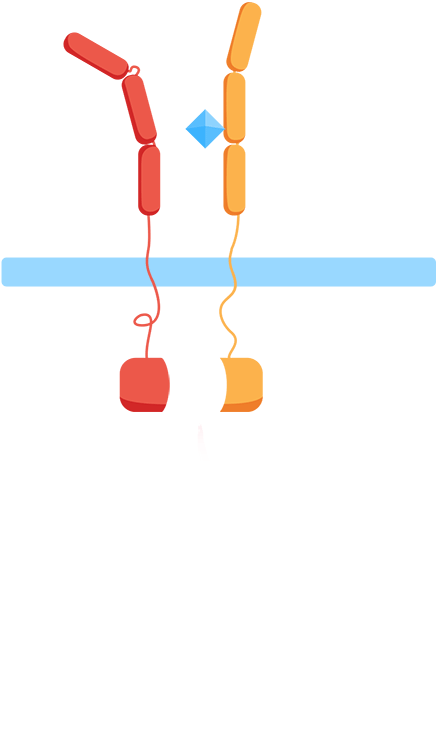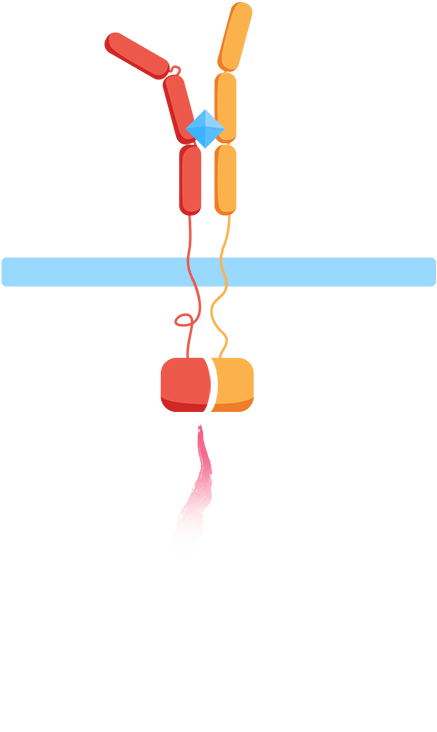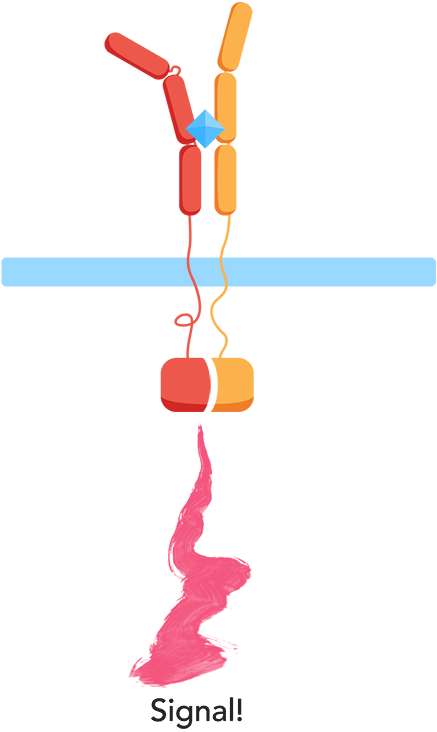
From the work we did in HP to understand CIDs and patients’ everyday problems and needs though, we knew we wanted to monitor biomarkers for general inflammation, interleukins, meaning that we'd have to develop our own receptor and signal transduction system in our yeast to sense them. At the same time, our human practices work also told us about the vast number of different interleukins, all important in different contexts, which influenced us to want to create a flexible platform enabling us to easily expand the biosensor to work with other interleukins. As most interleukin receptors are receptor tyrosine kinases, though, it means that luckily, most interleukin receptors function based on the same principle: association.
By using the extracellular portions of the human interleukin receptors, we would ensure an association of the extracellular domains in the presence of an interleukin, and by binding these to the membrane through transmembrane domains, we could couple the extracellular association of our two receptors to an intracellular association as well.
As such, the general mechanism of our biosensor would be:
From the work we did in HP to understand CIDs and patients’ everyday problems and needs though, we knew we wanted to monitor biomarkers for general inflammation, interleukins, meaning that we'd have to develop our own receptor and signal transduction system in our yeast to sense them. At the same time, our human practices work also told us about the vast number of different interleukins, all important in different contexts, which influenced us to want to create a flexible platform enabling us to easily expand the biosensor to work with other interleukins. As most interleukin receptors are receptor tyrosine kinases, though, it means that luckily, most interleukin receptors function based on the same principle: association.
By using the extracellular portions of the human interleukin receptors, we would ensure an association of the extracellular domains in the presence of an interleukin, and by binding these to the membrane through transmembrane domains, we could couple the extracellular association of our two receptors to an intracellular association as well.
As such, the general mechanism of our biosensor would be:
Mechanism
- 1. In the presence of the biomarker of interest, the extracellular receptor domains will associate.
- 2. The association of the extracellular domains will result in the intracellular complementation of a split protein.
- 3. Upon complementation, the signal is transduced and arrives at the nucleus.
- 4. Finally, a reporter gene is expressed, reflecting the initial level of interleukins.
Venturing deeper into this page will show you how we hope to achieve this by developing our very own receptor-systems!
Interleukin Receptors
This year, we’re simultaneously looking into three different interleukin receptors, each with their own unique properties, in order to sense IL-1α/β, IL-6 and IL-10.IL-1
IL-1
As mentioned above, the mechanism of action of IL-1 binding and signaling relies on the association of two or more receptors and the interleukin itself. The receptors in question are the IL-1R and the accessory receptor IL1RAcP. Formation of the heterotrimer and binding of the interleukin results in activation of the pathway in the native setting.Our first step when working with IL-1 was to look into the IL-1R family. Through our search, we found that there are some receptors that function as inhibitors of the IL-1 system, and that could be more useful in our context compared to the signaling IL-1RI receptor. Particularly, the IL-1RII decoy receptor was of interest to us. The IL-1RII receptor differs from the type 1 receptor in that it lacks an intracellular toll-like receptor essential for normal signal relay, but it has some nice features of interest to us. For example, it has lower affinity for the IL-1 antagonist compared to the type 1 receptor (Peters, Joesting, and Freund 2013), which is great for us, as it increases the selectivity for the signaling IL-1 molecules. It’s because of this increased sensitivity, and because the intracellular signaling domains are of no importance in our context, we decided to use the IL-1RII extracellularly.
IL-6
IL-6
Interleukin-6 is one of the most researched cytokines in inflammation source. It is an acute-phase mediator and generally functions pro-inflammatory in most tissues, even though it can also have anti-inflammatory effects. In humans, the mechanism for sensing IL-6 involves the heterotrimerization of the IL-6 receptor and the co-receptor glycoprotein 130 (gp13) with the IL-6 protein. Subsequently two of IL-6:IL-6R:gp130 trimers form a dimer. This interaction causes the intracellular parts of the two gp130 proteins to transphosphorylate each other and start a signaling cascade.We chose to try out two different design of our extracellular IL-6 sensing modules. In one design we link either halves of the intracellular split protein to the extracellular domains of the soluble isoform of gp130(sgp130) proteins while secreting a soluble isoform of IL-6R. Thus we utilize the association of two gp130 proteins in a heterohexamer in the first design. In the second design use the association of one gp130 and one IL-6R in a heterotrimer instead. Here we coupled the N-terminal half of the split protein to sIL-6R and the C-terminal half of the split protein to sgp130.
Glycoprotein 130 has six extracellular domains of which the three domains closest to the membrane mainly seem to function by positioning and bringing together the two gp130 proteins in the heterohexameric signaling complex. Since we do not depend on the association of the two gp130 proteins in the second design, we decided to use a truncated version of gp130 where we only use domain 1 to 3 in that design.
The sIL-6R has three domains; one Ig-like domain and two fibronectin-like type III domains. Only the two fibronectin-like domains (domain 2 and 3) seem to interact with the other proteins in the signaling complex(cite). We chose to use a truncated version of the sIL-6R were we only use domain 2 and 3 since we thought this would be easier to express in our chassis.
IL-10
IL-10
The receptors for IL-10 form heterotrimers like the receptors for IL-1. Here, the IL-10R type 1 associated with IL-10, and subsequently recruits IL-10R type II. The reason we decided to look into IL-10 is to have a non acute-phase cytokine in our system. This receptor is also much smaller than the previous two receptors, increasing the chance of correct folding in S. cerevisiae, and is located closer to the membrane compared to the IL-6 receptor for instance.Binding to the Membrane
The extracellular portions of the human interleukin receptors were then fused to a transmembrane domain to secure localization to the membrane, as previously mentioned. For this, we researched a lot of different endogenous yeast transmembrane domains (hereafter called TMDs) to find the one with the highest predicted localization to the membrane, based on sequence analyses. We ran a sequence analysis on 13 different endogenous single-pass type I transmembrane proteins as part of this endeavor, and using our knowledge of the characteristics of the phospholipid bilayer and transmembrane proteins in general, we came up with our own candidate for a transmembrane protein. This TMD would have hydrophobic amino acid residues pointing “inwards”, with the polar amino acid residues on either side and tryptophan in-between those areas.Our candidate had the following sequence of amino acids:
IAGIVIGVVLGVIFILIAILFAFW
And proved to have a higher predicted localization to the membrane than the TMDs we compared it to, as seen here:
INSERT PICTURE
As this added a level of unpredictability though, we decided to use the TMD Wsc1 for our project design henceforth, as some of our designs build on papers that use the Wsc1 domain (see later).
The next step was to imitate an intracellular transduction pathway, following the extracellular association of our receptor complexes. As all our interleukin receptors’ normal mechanisms of action build on two non-identical receptors associating, we thought to integrate a similar protein/protein interaction as the key part of our own transduction system. This gave rise to our first design: the split-ubiquitin design.
Three Tiers of Biosensor Designs
Click to go to the design sections!
Split Ubiquitin
This was the first design we developed, after being introduced to tools for identifying protein-protein interactions by our supervisor. Here we found the split-ubiquitin based Membrane Yeast Two-Hybrid method (MYTH) , where two different proteins of interest are fused to one half of a modified ubiquitin molecule each. The modifications made to the two halves of ubiquitin renders them unable to spontaneously bind to each other and reconstitute, without first being brought into close proximity of each other by another protein. This means that if the proteins of interest, that the ubiquitin halves are fused to, have a natural affinity for each other and consequently associate, the ubiquitin halves will also associate on the intracellular side of the membrane and reconstitute, as they’re being brought closer together by the association on the extracellular side. This suits our purposes perfectly, as we already know that the interleukin receptor parts that we’re using have a natural affinity for each other in the native setting (and of course in the presence of the fitting interleukin). Thus, by fusing ubiquitin to each of our receptors, we know that an extracellular association will result in an intracellular reconstitution of ubiquitin.
At the same time as we fuse the two halves of ubiquitin to our two receptor proteins, we fuse a transcription factor to the C-terminal part of ubiquitin. As ubiquitin is recognized by deubiquitinating enzymes upon reconstitution, this means that the deubiquitinating enzymes will cleave off the transcription factor bound to ubiquitin, resulting in the release of the free transcription factor into the cytosol and ultimately the cell nucleus, where it can exert its effect.

For our purposes, we’ve using a synthetic transcription factor developed by Dossani, Z. Y. et al. 2018. This consists of the bacterial LexA DNA binding protein, fused with the viral activator domain VP16. A corresponding hybrid promoter with operator regions replaced with sequences that are recognized by LexA is also used, to avoid transcription of yeast-native genes and ensure orthogonality .

However, as mentioned in the project description page, the concentrations of interleukins found in sweat are rather low, and as of now, this design has no real signal amplification step. On the other hand, this tried and tested method is a good, secure base to start working from.
Under normal circumstances we would’ve tested our design in the wet lab to gauge the importance of amplification at this point, or waited for the dry lab results to see if they confirmed our suspicions, but due to the restrictions in the lab we instead decided to re-think our design from the get-go. This meant that the next step for us would be to make another design, now with preferably more amplification.
Split TEV Protease and Membrane-bound Transcription Factor
Our intermediate design utilizes the same receptor-system as the ubiquitin-based design, but with few modifications. Thanks to our supervisor’s guidance, we were introduced to another kind of split-protein: the split TEV-protease. This was another method developed by Wehr, M. C. et al. In 2006 to monitor protein-protein interactions. Here, we again have two engineered inactive halves of the TEV-protease, that only regain activity when coexpressed as fusion constructs with interacting proteins . Therefore, we again utilize the receptor/TMD domains from the previous designs, but now each of our receptors will be fused to one half of the TEV-protease instead with a flexible linker.
In parallel, we also express the Wsc1 TMD, which will be sorted and localized to the membrane. To this TMD, we’ll fuse the same transcription factor from the previous design (LexA-VP16), and use the recognition sequence for the TEV-protease as the linker between the two. This means that the TEV-protease, upon reconstitution, will be able to cleave the transcription factor and free it into the cytosol. In theory, the TEV-protease will be able to cut many transcription factors loose, meaning that one interleukin (by extension of the association of our two receptors) will result in the cleavage of multiple transcription factors and thus an amplification of the signal.

Again, this extra amplification step made us hopeful, but in order to achieve the highest level of amplification possible we moved on to other venues.
Simultaneously, we started modeling our pathways in the dry lab.
Hijacking the Pheromone Pathway
Our last and most ambitious design hinges on hijacking the pheromone pathway in yeast. We had no doubt that this would give us the most amplification, which is especially important in our case given the low concentrations of interleukins in sweat (Hladek et al. 2018; Dai et al. 2013). The pheromone pathway is XXXXX EXPLAIN HERE OR NO?
In order to hijack the pheromone pathway, we initially set out to understand
how the Ste5 scaffold protein was recruited to the membrane by the beta/gamma
complex. Soon though, we saw that the interaction between the beta/gamma
complex and the scaffold was more complicated than previously thought, and
introducing changes to this step seemed risky (SOURCE about how we don’t
know what beta/gamma does exactly here). Since the exact mechanism by which
the beta/gamma complex recruits Ste5 was unknown to us, we decided to take
a more conservative approach instead of changing beta/gamma for another
recruiting maneuvre. Here, we finally thought to introduce an inhibitory
sequence on beta, that could be removed at will, so as to keep the beta/gamma
complex as the recruiting element.

This inhibitory sequence would then,
building onto the previous design, contain the TEV protease cleavage site,
so an extracellular signal could trigger the TEV protease to cut off the
inhibitory sequence and start the signaling. The next step from here would
be to design such an inhibitory sequence, and through talking to our
supervisor, we agreed that we should stay in the same conservative vein
and look at natural inhibitors of the signaling. Here, the alpha subunit
of the G protein was an obvious choice, as the alpha subunit binds to
beta/gamma in the absence of an extracellular signal, and hinders them
from recruiting Ste5 and completing the pheromone pathway signaling.
As a natural inhibitor, G alpha was a great choice for us.
As a natural inhibitor, G alpha was a great choice for us.
The next step from here was to find places to insert the TEV protease
cleavage sites in G alpha. Our goals when doing this was to have a G alpha that
For this, we had many approaches. Our first thought was to look at the yeast G alpha’s sequence, and change very few amino acids in places that already resembled the TEV recognition site. During this first iteration, we used the following recognition site:
EXLYΦQ\φ where X is any residue, Φ is any large or medium hydrophobic and φ is any small hydrophobic or polar residue .
(INSERT PRELIMINARY RESULTS?)
However, we found that introducing different amino acids (albeit with the same properties) in the different cleavage sites would prove to make subsequent wet lab troubleshooting harder, as well as change the dry lab output in unreasonable ways, as the TEV protease would then have different efficiencies depending on the used recognition site (Dougherty et al., 1989). In order to limit the number of unknowns, and to have the same affinity of our TEV protease to our recognition site, we opted to use the same recognition site everywhere; ENLYFQG.
Using mutagenesis results from UniProt and other literature ( Gladue and Konopka 2008) we found the residues that have been found to give constitutive activity in the pheromone pathway as a result of alpha not being able to bind to beta/gamma (Gladue and Konopka 2008) etcetera, and through extensive computer modeling described under our dry lab section, we eventually landed on some G alpha mutants where the TEV recognition site is inserted into tactical, most promising areas that should not interfere with normal G alpha function. For details, check out our engineering success page!
So, to summarize, our last and final design entails:
- 1. could keep its GTPase activity, so it could be used in other contexts in the future.
- 2. could bind to and exert an inhibitory function on the beta/gamma complex’s ability to recruit Ste5.
- 3. could, once cut by the TEV protease in the presence of a signal, dissociate from beta/gamma again, enabling regular signaling and recruitment of Ste5.
For this, we had many approaches. Our first thought was to look at the yeast G alpha’s sequence, and change very few amino acids in places that already resembled the TEV recognition site. During this first iteration, we used the following recognition site:
EXLYΦQ\φ where X is any residue, Φ is any large or medium hydrophobic and φ is any small hydrophobic or polar residue .
(INSERT PRELIMINARY RESULTS?)
However, we found that introducing different amino acids (albeit with the same properties) in the different cleavage sites would prove to make subsequent wet lab troubleshooting harder, as well as change the dry lab output in unreasonable ways, as the TEV protease would then have different efficiencies depending on the used recognition site (Dougherty et al., 1989). In order to limit the number of unknowns, and to have the same affinity of our TEV protease to our recognition site, we opted to use the same recognition site everywhere; ENLYFQG.
Using mutagenesis results from UniProt and other literature ( Gladue and Konopka 2008) we found the residues that have been found to give constitutive activity in the pheromone pathway as a result of alpha not being able to bind to beta/gamma (Gladue and Konopka 2008) etcetera, and through extensive computer modeling described under our dry lab section, we eventually landed on some G alpha mutants where the TEV recognition site is inserted into tactical, most promising areas that should not interfere with normal G alpha function. For details, check out our engineering success page!
So, to summarize, our last and final design entails:
- 1. A signaling interleukin reaches our two receptors.
- 2. The receptors associate extracellularly, giving rise to the intracellular complementation of a split TEV protease.
- 3. The now active TEV protease can cut a mutant G alpha protein into smaller peptide fragments, that’ll dissociate from the beta/gamma complex.
- 4. The beta/gamma complex can exert its recruiting function on the Ste5 scaffold protein and recruit it to the membrane, thus triggering the pheromone cascade, eventually resulting in the phosphorylation of our modified LexA-Ste12 transcription factor.
- 5. Our activated, phosphorylated transcription factor LexA-Ste12 will bind to the synthetic promoter 6xlexo eno1, and result in the transcription and translation of our reporter protein.

This last and final design has a lot of strengths compared to the two prior designs, but also some drawbacks. Of course, changing such a conserved protein is a great challenge, as any small change could result in great consequences for its conformation and normal functions. At the same time, however, this design proved to be the best in terms of amplification and sensitivity, as shown through our modeling of the system compared to the two others.
For this reason, and to check whether our dry lab results fit with reality, all three of the abovementioned designs were tested in the wet lab, the results of which can be found here!
HOW DO WE SAY THAT WE USED THE ENGINEERING CYCLE PERFECTLY, AND WHERE DO I PUT MY FIGURE???????
- Zola H. Analysis of receptors for cytokines and growth factors in human disease. Dis Markers. 1996;12(4):225-240. doi:10.1155/1996/807021 ↩
- Gunde T, Barberis A. Yeast growth selection system for detecting activity and inhibition of dimerization-dependent receptor tyrosine kinase. Biotechniques. 2005;39(4):541-549. doi:10.2144/000112011↩
- Snider, J., Kittanakom, S., Curak, J., & Stagljar, I. (2010). Split-ubiquitin based membrane yeast two-hybrid (MYTH) system: A powerful tool for identifying protein-protein interactions. Journal of Visualized Experiments. https://doi.org/10.3791/1698↩
- Dossani, Z. Y., Reider Apel, A., Szmidt-Middleton, H., Hillson, N. J., Deutsch, S., Keasling, J. D., & Mukhopadhyay, A. (2018). A combinatorial approach to synthetic transcription factor-promoter combinations for yeast strain engineering. Yeast. https://doi.org/10.1002/yea.3292/↩
- Wehr, M. C., Laage, R., Bolz, U., Fischer, T. M., Grünewald, S., Scheek, S., Bach, A., Nave, K. A., & Rossner, M. J. (2006). Monitoring regulated protein-protein interactions using split TEV. Nature Methods. https://doi.org/10.1038/nmeth967↩
- Nallamsetty, S., Kapust, R. B., Tözsér, J., Cherry, S., Tropea, J. E., Copeland, T. D., & Waugh, D. S. (2004). Efficient site-specific processing of fusion proteins by tobacco vein mottling virus protease in vivo and in vitro. Protein Expression and Purification. https://doi.org/10.1016/j.pep.2004.08.016/↩
- Dai, X., Okazaki, H., Hanakawa, Y., Murakami, M., Tohyama, M., Shirakata, Y., & Sayama, K. (2013). Eccrine Sweat Contains IL-1α, IL-1β and IL-31 and Activates Epidermal Keratinocytes as a Danger Signal. PLoS ONE. https://doi.org/10.1371/journal.pone.0067666/↩
- Dougherty, W. G., Cary, S. M., & Dawn Parks, T. (1989). Molecular genetic analysis of a plant virus polyprotein cleavage site: A model. Virology. https://doi.org/10.1016/0042-6822(89)90603-X↩
- Gladue, D. P., & Konopka, J. B. (2008). Scanning mutagenesis of regions in the Gα protein Gpa1 that are predicted to interact with yeast mating pheromone receptors. FEMS Yeast Research. https://doi.org/10.1111/j.1567-1364.2007.00311.x/↩
- Hladek, M. D., Szanton, S. L., Cho, Y. E., Lai, C., Sacko, C., Roberts, L., & Gill, J. (2018). Using sweat to measure cytokines in older adults compared to younger adults: A pilot study. Journal of Immunological Methods. https://doi.org/10.1016/j.jim.2017.11.003/↩
- Peters, V. A., Joesting, J. J., & Freund, G. G. (2013). IL-1 receptor 2 (IL-1R2) and its role in immune regulation. In Brain, Behavior, and Immunity. https://doi.org/10.1016/j.bbi.2012.11.006/↩

