
Categories
Design Overview
In our mission to create a comprehensive solution to PF, we decided to achieve three goals as following: 1. Combine pathway-blocking proteins with collagen-degenerating proteins to reverse PF course 2. Design an appropriate drug carrier system to make the administration route more accurate and more convenient. 3. Establish a protein drugs’ efficient platform providing a methodology for saving more people suffering from fibrosis disease.
Introduction
Pulmonary Fibrosis(PF) is a widespread and persistent disease. Myofibroblasts and TGF- associated signaling pathways play a critical role in the development of fibrosis.In order to create a comprehensive solution to the disease of organ fibrosis, the breakthrough point is the common and difficult to solve pulmonary fibrosis. We hope to create an effective treatment model for organ fibrosis that can be widely replicated and used as a template for treatment of pulmonary fibrosis.
In this case, we designed two treatment modules.
For one thing, we want to block the cell signaling pathway that induces pulmonary fibrosis to avoid the increase of fibroblasts. Thus the further deepening of pulmonary fibrosis can be inhibited.
For another, appropriate degradation of collagen is essential for fibrosis focus therapy, as to improve tissue compliance and restore the normal tissue function.
Due to the special structure and function of the lungs, in which the oxygen exchanging space and the ultrathin, elastic and capillary rich tissue structure are required, we made a challenging work to make the use of treatment and therapy more precise and controllable. And if our treatment can be applied in pulmonary fibrosis, we believe that it will be applied in other diseases, such as cirrhosis and renal fibrosis, and save more patients’ lives.
Protein Design
In our study, we designed a variety of fusion proteins targeting the pulmonary fibrosis region to block the fibrosis signaling pathway. These proteins are made up of three main parts:
- The head is the fibrosis treatment-related domain.(1.signal blocking & 2.collagen degeneration)
- The rigid linker stabilizes the protein structure and separate two functional domains.
- The tail is the domain which can recognise and target nidus.
So fusion protein firmly binds to the nidus by the tail then blocks the activation signaling pathway of myofibroblasts or degradation of excessive collagen. These fusion proteins are packaged in the liposomes. And they will act directly on the lesions by aerosolizing and inhaling the lungs.

Fig1. The two steps while our drugs dealing with pulmonary fibrosis
Signal Blocking Domain (head A)
TGF-β truncated Receptor
TGF-β is a key factor in triggering fibrosis by inducing the activation and differentiation of myofibroblasts, which leads to excessive production of extracellular matrix then forming fibrosis in organs or tissues.

Fig2. Schematic diagram of the mechanism of pulmonary fibrosis formation
After modeling evaluation, we select M7824 rather than other 3 peptides (domains form P144, TMED10 and one unnamed peptide) as TGF-β blocking domain.
M7824 consists of an IgG1 targeting programmed death ligand 1 (PD-L1) moiety fused via peptide linkers to the extracellular domain of two TGF-β receptor II molecules designed to ‘trap’ TGF-β. It has been used in the treatment of cancer and achieved remarkable results and its active region has been proved to be efficient.
Consequently, we selected the active domain to capture TGF-β, as one of the active regions of drug proteins, so that TGF-β can be inactivated. Therefore, the course of fibrosis can be interrupted.
PDGFβ truncated Receptor
Mononuclear cells are usually present in lung fibrosis. Activated monocytes and macrophages in culture have been shown to produce several growth factors including platelet-derived growth factor (PDGF). PDGF is a potent mitogen and chemoattractant for fibroblasts and smooth muscle cells and a stimulator of collagen synthesis. Therefore, PDGFβ truncated receptor also has therapeutic potential for pulmonary fibrosis.[1]
Plasmin Cascade Activating Domain (head B)
Why plasmin activation can be applied in fibrosis treatment?
Plasmin has been used in the treatment of thrombosis. As a proteolytic enzyme, plasmin is also promising in applications in terminal pulmonary fibrotic diseases.
Transgenic animal experiments have firmly established a causal link between the level of activated plasmin and the severity of fibrosis. In vitro, plasmin directly activates proMMP-1, proMMP-3, proMMP-9, proMMP-10, and proMMP-13. Membrane type-1 MMP converts the 72-kDa proMMP-2 to anintermediate 64-kDa species, which is activated by plasmin following conversion to a 62-kDa molecule . Plasmin induces remodeling of extracellular matrix by the release of modulating molecules like HGF, PGE2 and inducing anoikis of fibroblasts.
How to activate plasminogen gently?
In consideration of controlling side effects, we did not choose plasminogen activating peptides with enzymatic activity, but chose peptides with carboxyl terminal lysines. Among the peptides with carboxyl terminal lysines, the α2-antiplasmin C-terminal 26 peptide was selected.
A2-Antiplasmin (a2AP) interferes with the binding of plasminogen to fibrin because lysine residues in its carboxy-terminal region compete with those in fibrin. While this overall process causes an inhibition of fibrinolysis, the converse was observed with a 26-residue synthetic peptide (AP26) corresponding to the carboxy-terminal region of a2-Antiplasmin . Specific lysine residues in the carboxy-terminal 26-amino acid sequence (AP26) of a2AP comprise the initial plasmin(ogen)-binding site on a2AP . The synthetic AP26 peptide competitively inhibit the interaction between plasmin and fibrins by blocking lysine-binding sites of plasmin while not affecting its catalytic site . The synthetic peptide reduces plasminogen’s ability to bind to other substrates with lysine residues, but promotes plasminogen activation.
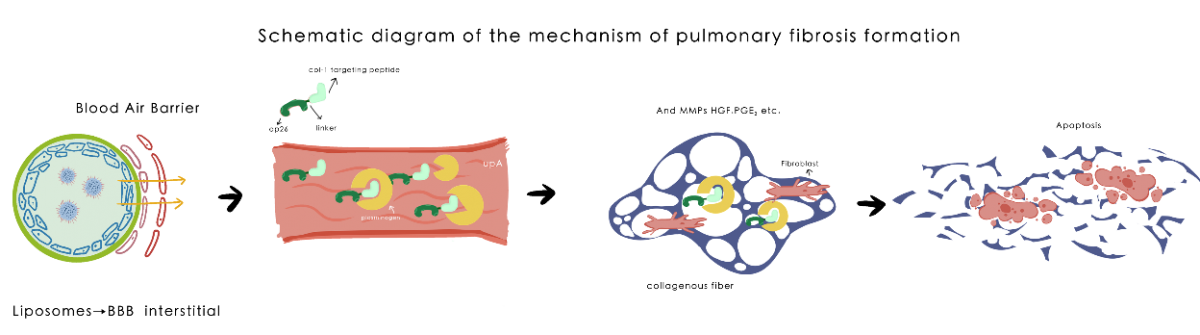
Fig3. Mechanism diagram of fibrinolytic enzyme activator peptides
How to control its side effect?
In order to control side effects and avoid severe diseases such as DIC or thrombosis,
(1) We chose a relatively mild activating peptide with dual effects, which promotes allosteric activation of plasminogen and has a competitive inhibitory effect on activated plasmin;
(2) We chose a highly selective collagen targeting peptide to control the scope of effect by enriching the drug in the lesion;
(3) Reducing the required drug dosage through pulmonary administration by liposomes (selection process provided in our modeling page)
(4) Combine with plasminogen inhibitors (such as Trasylol, etc.) in dangerous situations.
We hope to push the balance from ECM synthesis to slight ECM degradation through targeted delivery of small doses of drugs. Since the lung is functionally redundant, we do not expect that this solution can degrade all interstitial collagen depositions, but we can improve the compliance of the whole lung, stop the positive feedback loop of pulmonary fibrosis, and realize the adjustment and reshaping of the pulmonary microenvironment. In this way we may allow the balance of ECM synthesis and degradation to return to a balanced position.
Focus Targetting (the tail)
In order to make the drug protein adhere to the pulmonary fibrosis nidus, the collagen over-proliferation and rich area, more stably, three different peptide chains with high collagen affinity :
Type 4: Type 4 is a propolypeptide of von Willebrand factor (pp-vWF) binding to type I collagen, and the binding site was defined to the sequence as follows: WREPSFCALS, which we called Type 4. It is used to develop a collagen‐based wound targeting repair system, two collagen‐binding domains (CBDs) into the human basic fibroblast growth factor (bFGF) and has been shown to have a good therapeutic effect.
CLIY: CILY is a short peptide derived from Collagen I receptor, which can be used to target Collagen type I and has a very high affinity. Its sequence is RRANAALKAGELYKCILY.
SILY: SILY is a derivative of CILY. At present, it has been applied as a targeted probe in the treatment of skin scarring and the regulation of collagen fiber formation, and achieved significant efficacy.
: HL5, a kind of linker peptide, a part of a peptide between relatively rigid structural domains, which can be helpful when highly specific spacing between domains must be maintained.
Probes were selected to connect with the TGF- affinity active region, M7824, by the rigid amino acid Linker sequence HL5, so as to ensure that the two active sites maintain their original structure and biological activity. Such a design could give the drug protein a more precise therapeutic effect, and prevent the drug from spreading into the blood and causing disruption of cellular signals in normal tissues.
Drug Delivery System
We encapsulate the drug proteins into liposomes and make aerosols. In this case, liposome agents will be inhaled into the lungs in the form of aerosols to achieve a local high concentration, limiting the scope of drug action from a general level, and improving drug targeting.
Furthermore, we selected highly specific molecules of pulmonary fibrosis lesions as targets through screening, designed targeted probes and embedded them into liposome bilayer membrane, so as to improve the targeting of lesions from the molecular level. Finally, we also optimized the size of the liposome to improve its penetration of ECM by adjusting its radius, thus ensuring that it can spread from the outer surface of the alveoli to the inner pulmonary capillaries to move with the microcirculation to the focal area, and then firmly binding.
These measures lead to a concentrated and specific distribution of drugs in areas of pulmonary fibrosis.

Fig4. Schematic diagram of drug system assembly
Screening Platform
Sequence Selection Stage
- Select potential domains from related articles.
- Make different combinations then evaluate their function with Z-DOCK, I-TASSER and pymol, choose the best ones.
- Stimulate then assess their metabolism in vitro with Gastroplus, choose the easily metabolic ones.
Expression Stage
1. Cell-free protein synthesis has been applied to produce slight protein which can be applied for verification experiments in vivo.

Fig5. Schematic diagram of cell-free protein synthesis
2. E. coli fermentation has been applied to produce adequate protein for thorough verification experiments (in animals and human in the future).
Verification Stage
1. In molecular level, we will use Surface Plasmon Resonance (SPR) to evaluate their binding function with targeting signal or focus as kD value shown.
2. In cell level, we used HFL-1 cell line (induced by TGF-beta1) to verify the drugs’ function with
(1) Reverse transcription quantitative polymerase chain reaction(RT-qPCR to assess Reverse transcription polymerase chain reaction
(2) Immunocytochemistry(ICC) to verify whether the collagenous fiber can be degenerated efficiently.
3. In organoid level, we will evaluate whether our drugs can improve air exchanging at steps as follows,
(1) Establish infinite element model with material parameters of Polyurethane/small intestinal submucosa(PU/SIS) then select the best models, the mechanical parameters of which are most similar to aevoli.
(2) Establish infinite element model with material parameters of Polyurethane/small intestinal submucosa(PU/SIS) then select the best models, the mechanical parameters of which are most similar to aevoli.
(1) Establish infinite element model with material parameters of Polyurethane/small intestinal submucosa(PU/SIS) then select the best models, the mechanical parameters of which are most similar to aevoli.




Reference