Overview
For the project S-POP, we decided to divide the parts into more manageable tasks and experiments. The two major parts of S-POP contained in the MFC, is the E.coli biosensor module and the S. oneidensis oscillation module. To demonstrate the feasibility of S-POP as a project we've highlighted the successful parts which are vital to the function of the system.
Sanger sequencing was utilized to show successful insertion of desired gene constructs. To show the expression and activation of the different constructs, fluorescence tags (GFP, mCherry) and Myc-tags were used. The final S. oneidensis oscillation model was developed with the aid of numerical models and simulations. Voltage measurements were used to calculate the current production of the results from the MFC.
Biosensor
The modular biosensor relies on a promoter region that is upregulated by the presence of specific pollutants. When the pollutant sensing parts are activated, a quorum sensing (QS) molecule, N-acyl homoserine lactone (AHL), is produced.
Successful gene insertion
Sanger sequencing was performed to evaluate if the genes of interest had been introduced into E. coli. Sequencing results showed that the vital sensing parts BBa_K3440005 (prmA-LuxI-myc tag) and BBa_K3440018 (constitutive promoter-bphR2 - myc tag - promoter bphR1-LuxI) were successfully inserted. These regions include the pollutant sensing parts (prmA and bphR1/2) as well as the QS-signal producing part (LuxI). In total 12 constructs were found to have the right alignments

Figure 1: Sequence comparison for construct O (top) and F (bottom)
Gene expression
After the successful insertion of the gene fragment, it was essential to test the protein expression. The essential QS-molecule produced by the gene LuxI, under the constitutive promoter, was successfully expressed in E. coli as shown by Western Blot results.
Viability
To ensure of the E. coli biosensor module viability, tests with the specific pollutant in varying concentrations were performed. These viability assays started with similar levels to what could be found in the environment, but also higher pollutant concentrations were utilized. E. coli was not severely affected by the pollutants.

Figure 2: Growth curves of Top 10 E. coli BL21, constructs F and plasmid 3.2 with PFOS and biphenyls
Pollution detection
After viability assays showing that E. coli can survive with the chosen pollutants present, the inducibility of the promoters was checked. Tests with similar pollution levels found in the environment and the high levels neither induced the promoters.
S. oneidensis oscillation
E. coli successful gene insertion
Shewanella oneidensis requires conjugation or electroporation for optimal gene insertions. The following gene insertions were performed with E. coli as a representative organism. Firstly, the oscillation gene circuit insertion in E. coli was evaluated by Sanger sequencing. The parts, BBa_K3440014 (Constitutive promoter- LuxR- myc tag-Inducible Promoter Lux-GFP) and BBa_K3440012 (Constitutive promoter- RhlR- myc tag- inducible Promoter Rhl-GFP) respectively, were successfully inserted in E. coli and characterized. These parts are required for the oscillation to occur, with the exception of the GFP and myc-tag which was used to evaluate protein expression. Furthermore, LuxR and RhlR could be induced by AHL and/or constructs expressing LuxI and RhlI. These results (i.e. the successful expression of LuxI and RhlI in co-cultures and with no synthetic AHL) suggests successful binding to LuxR and RhlR respectively and induction of specific promoters, which is shown by expression of GFP. The results of this functionality assay are supported by the Western blot results, which showed the expression of Myc-tagged Lux, RhlI and LuxR.

Figure 3: WB results of constructs
Modelling / simulations
A vital part of S-POP is the S. oneidensis oscillation-system within the microbial fuel cell. Due to complications stemming from COVID-19, we were not able to test the system on the bench. However, we showed success in modelling the system. After running models with generic kinetic values, a representative model was produced. These models evaluate different conditions and simulate applicable variations. The Model showed that an oscillation can be obtained and can therefore be used to distinguish and identify different pollutants depending on the period of the oscillations.

Figure 4: Improved inducible oscillatory circuit at induction point T=55
Molecular dynamics
One crucial part of the oscillatory system was the protein AiiA, which is a lactonase that degrades AHLs. To determine the affinity of the lactonase, docking was performed using molecular dynamics, and the energies used to determine kinetic constants to the different AHLs used.

Figure 5: HL4-ideal in the binding pocket of AiiA
MFC
The electric output from the microbial fuel cell (MFC) relies on S. oneidensis forming a biofilm on the electrode. MtrB is a vital part of the biofilm attachment as well as the outer membrane electron transport that facilitates the redox reaction which creates the electric output. In the S. oneidensis wild strain MtrB is active and in the S. oneidensis MtrB-knockout is inactivated.
S. oneidensis wild type
When the S. oneidensis wildtype-strain was introduced into the MFC, a biofilm was clearly established. The voltage measurements also indicated steady electric outputs.

Figure 6: Measurement of current over time in red, with the different phases in cyan
S. oneidensis MtrB-knockout
On the other hand, when the S. oneidensis MtrB-knockout strain was introduced to the MFC under the same conditions as the wildtype, no biofilm was formed. The voltage measurements affirmed that the MtrB-knockout strain has a significantly lower electric output.

Figure 7: Measurement of current over time in cyan
The current production by the different strains are illustrated in Figure 8. These results indicate that when MtrB is inactivated in S. oneidensis, a decrease in voltage can be measured. By utilizing the MtrB-gene, an oscillatory curve can be produced from the activation/repression of the promoter as suggested by the modelling of the system.
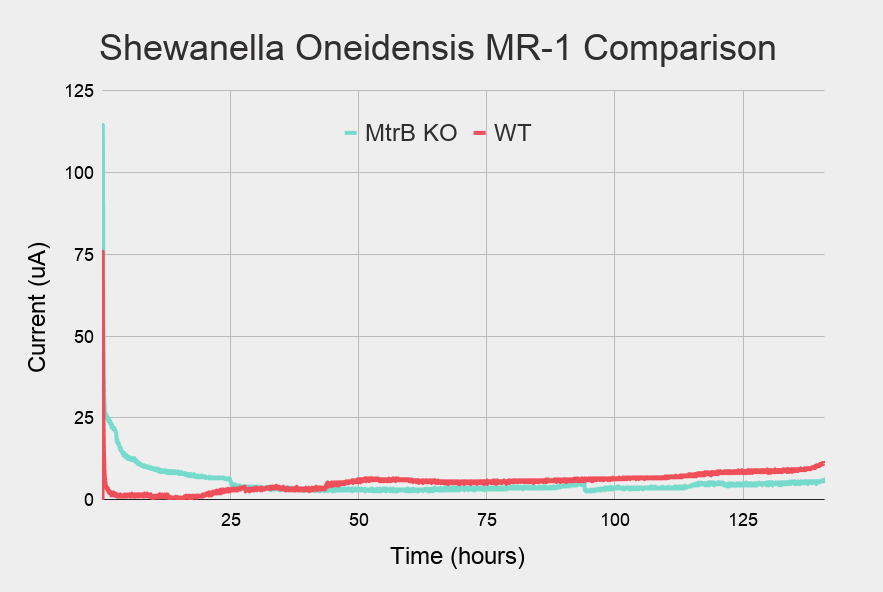
Figure 8: Comparison between the initial part of the WT current (in red) and the KO current (in blue) over time
Current production over time, in MFC with S. oneidensis Wild Type (pink), MtrB KO (light blue)
Conclusion
The initial plan was that all the separate components would be merged into a functioning system after optimization and characterisation. However, this was not possible due to lack of time. With the foundation built by literature research and laboratory work, a prototype of the modular biosensor could be developed and tested. Even though not all components have been tested, the current findings show promising results.
In the future, the biosensor module could be modified to detect other compounds as well, after appropriate adjustments of the system. The main challenge in the future will be to optimise the oscillation circuit to perform more accurate measurements of the levels of compounds in the sample.

