Results
Sample processing
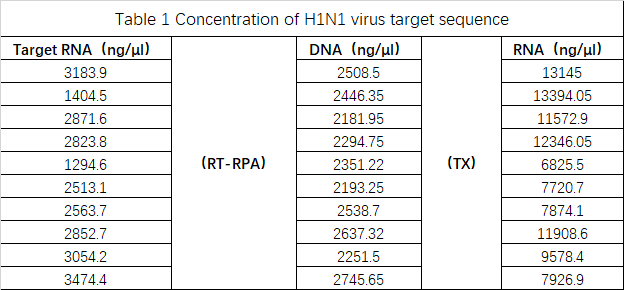
Result:
To simulate the state in which the viral nucleic acid is wrapped in a protein shell under real conditions, a plasmid with a viral target sequence was constructed and introduced into E. coli BL21. Then the recombinant E. coli was cultivated and induced to transcribe the target RNA. The resulting cells were used to simulate pathogens for subsequent amplification experiments. It provides an important reference for patient sample detection with the device.
The results were summarized in Table 1. The data was originally intended to be used for modeling, but accurate data could not be obtained due to the instrument and operation reasons. Therefore, the modeling failed, and the experiment can be improved after subsequent improvements.
Cloning
PCR amplification and purification of His-SUMO tag
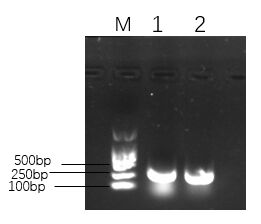
Figure 1. The agarose gel electrophoresis of His-SUMO tag.
Note:
Lane M:Marker DL2000
Lane 1 and Lane 2:His-SUMO tag1. PsmCas13b
Result:
As shown in Figure 1,in the range of 250-500 bp, there was a bright band in the product, which demonstrated that the His-SUMO fragment was amplified successfully. The His-SUMO tag concentration was shown in Figure 2.
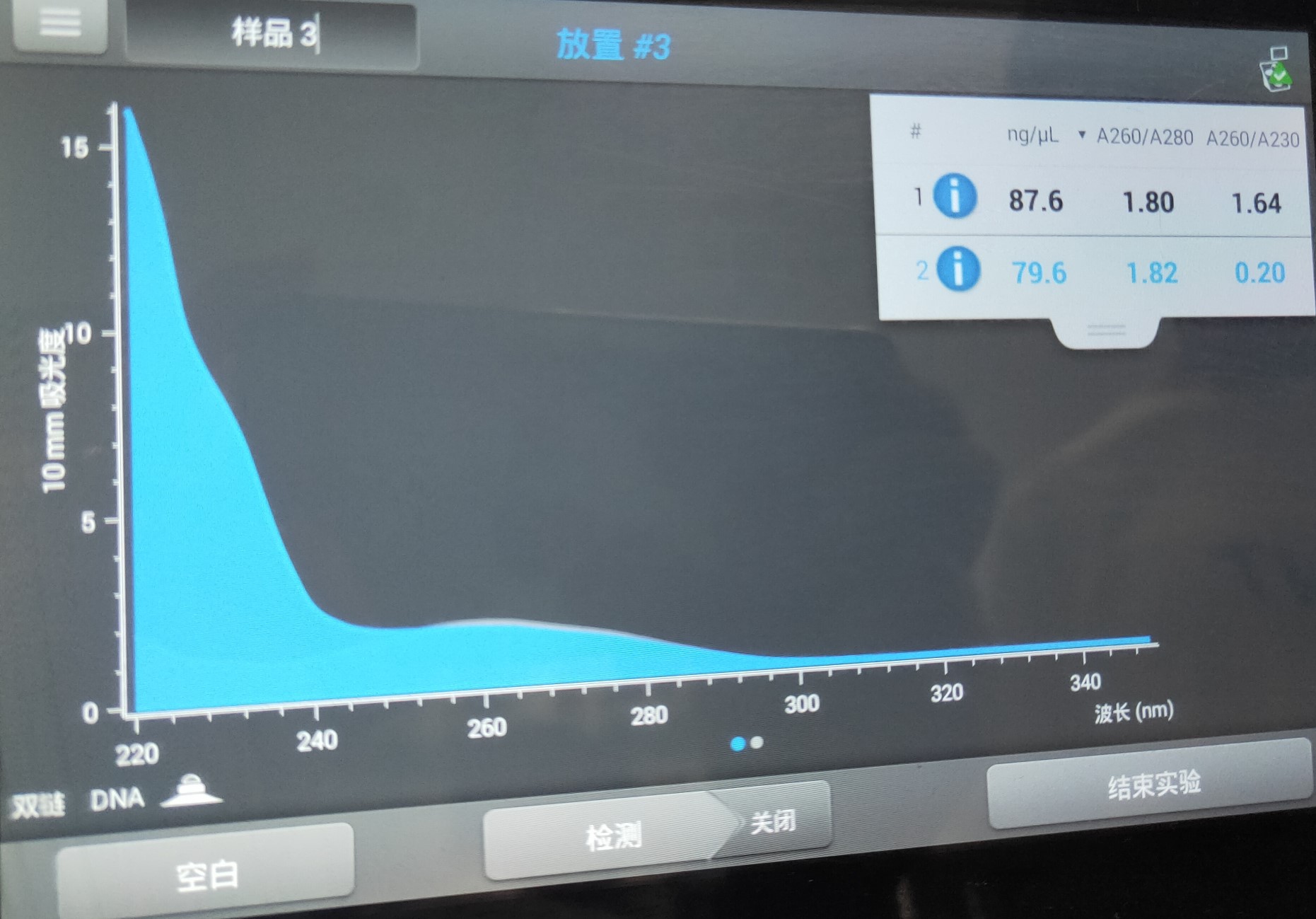
Figure 2. His-SUMO tag concentration.
1. PsmCas13b
Linear amplification and purification of Psm plasmid

Figure 3. The agarose gel electrophoresis of Psm plasmid.
Note:
Lane M:Marker 1kb plus
Lane 1 and Lane 2:Psm plasmid
Result:
As shown in Figure 3,there were bright bands in the range of 8000 to 10000 bp, so the Psm plasmid was linearized and amplified successfully. The Psm plasmid concentration was shown in Figure 4.

Figure 4. Psm plasmid concentration
The recombinant plasmid His-SUMO-cas13b-Psm was transferred into DH5a
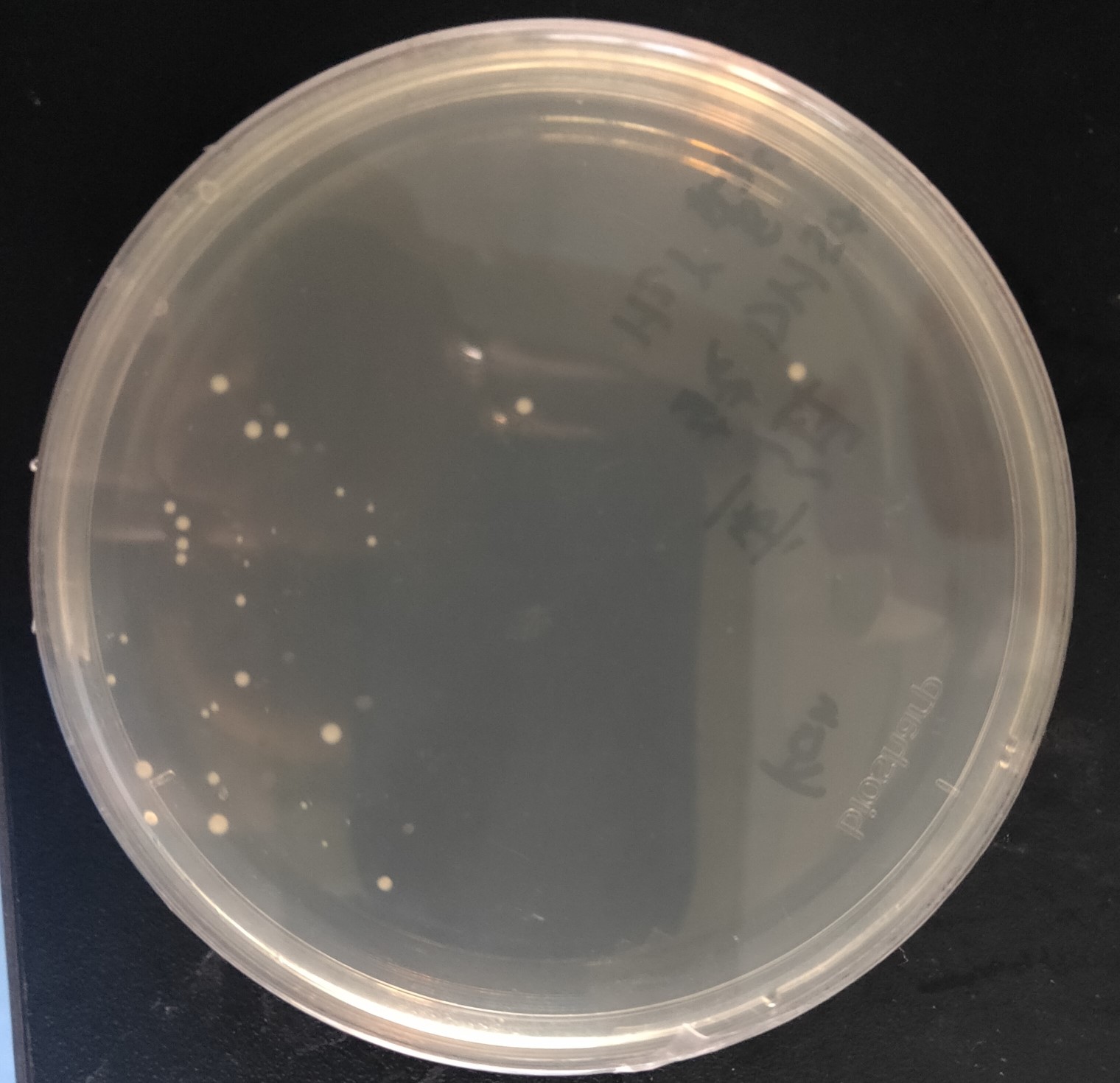
Figure 5. The transformants of recombinant plasmid His-SUMO-cas13b-Psm
Result:
As shown in Figure 5, transformant colonies were found in the Ampicillin resistant medium.
Identification of recombinant plasmid SUMO-cas13a-Psm by colony PCR
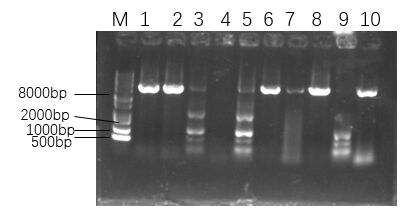
Figure 6. The agarose gel electrophoresis of His-SUMO-cas13b-Psm transformants.
Note:
Lane M:Marker 1kb plus
Lane 1 to Lane 10:His-SUMO-cas13b-Psm transformants
Result:
As shown in Figure 6, among the selected single colonies, only the first, second, sixth, eighth and tenth single colonies had bright bands in the range of 8000 to 10000 bp, which demonstrated that the recombinant plasmid was successfully transformed.
2. CcaCas13b
Linear amplification and purification of Cca plasmid

Figure 7. The gel electrophoresis of Cca plasmid.
Note:
Lane M:Marker 1kb plus
Lane 1 to Lane 4:Cca plasmid
Result:
As shown in Figure 7, there were bright bands in Lane 1 and Lane 4 in the range of 8000 to 10000 bp, which demonstrated that the Cca plasmid was linearized and amplified successfully. The Cca plasmid concentration was shown in Figure 8.
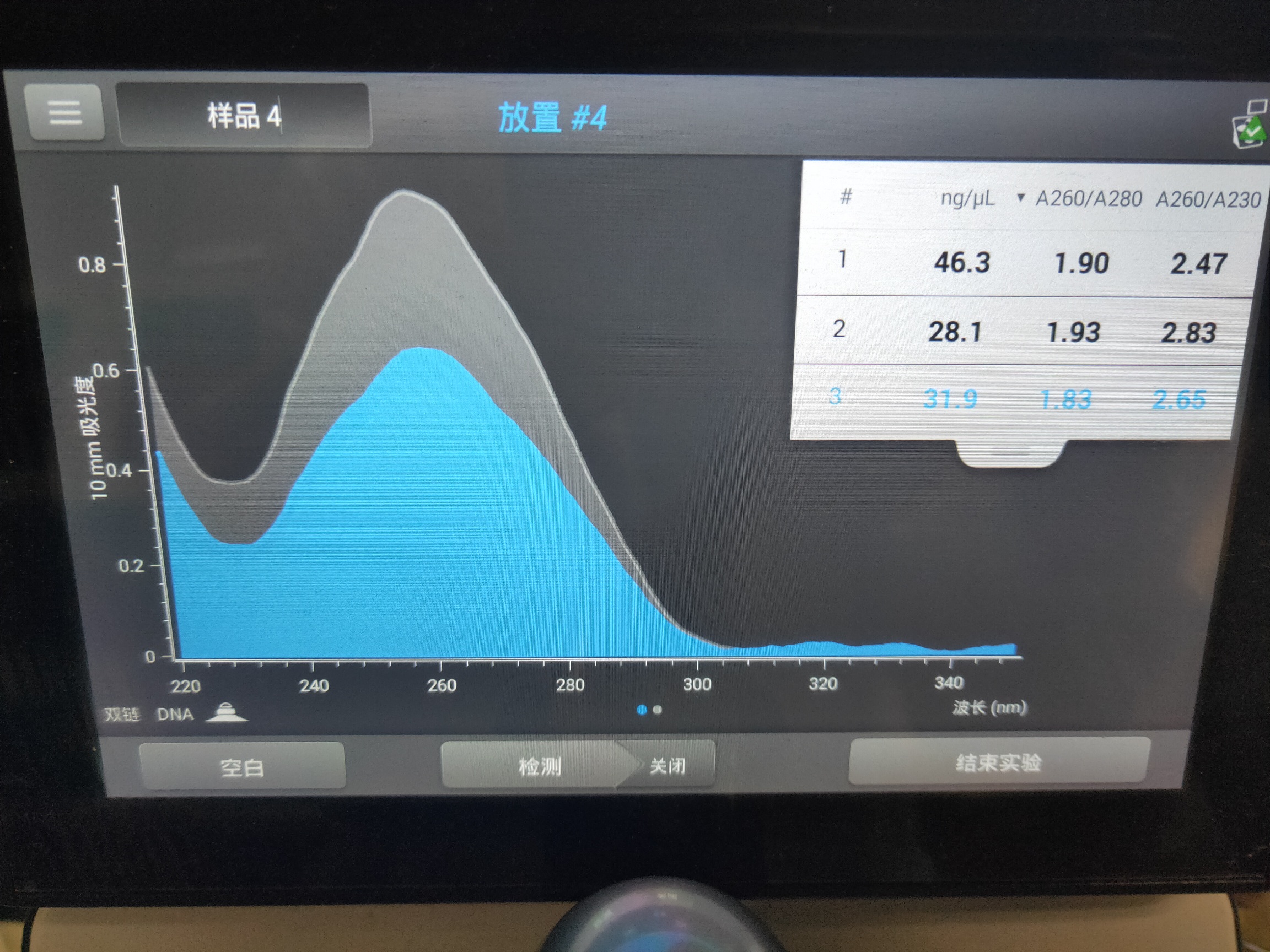
Figure 8. Cca plasmid concentration
The recombinant plasmid His-SUMO-CcaCas13b was transferred into E. coli DH5a
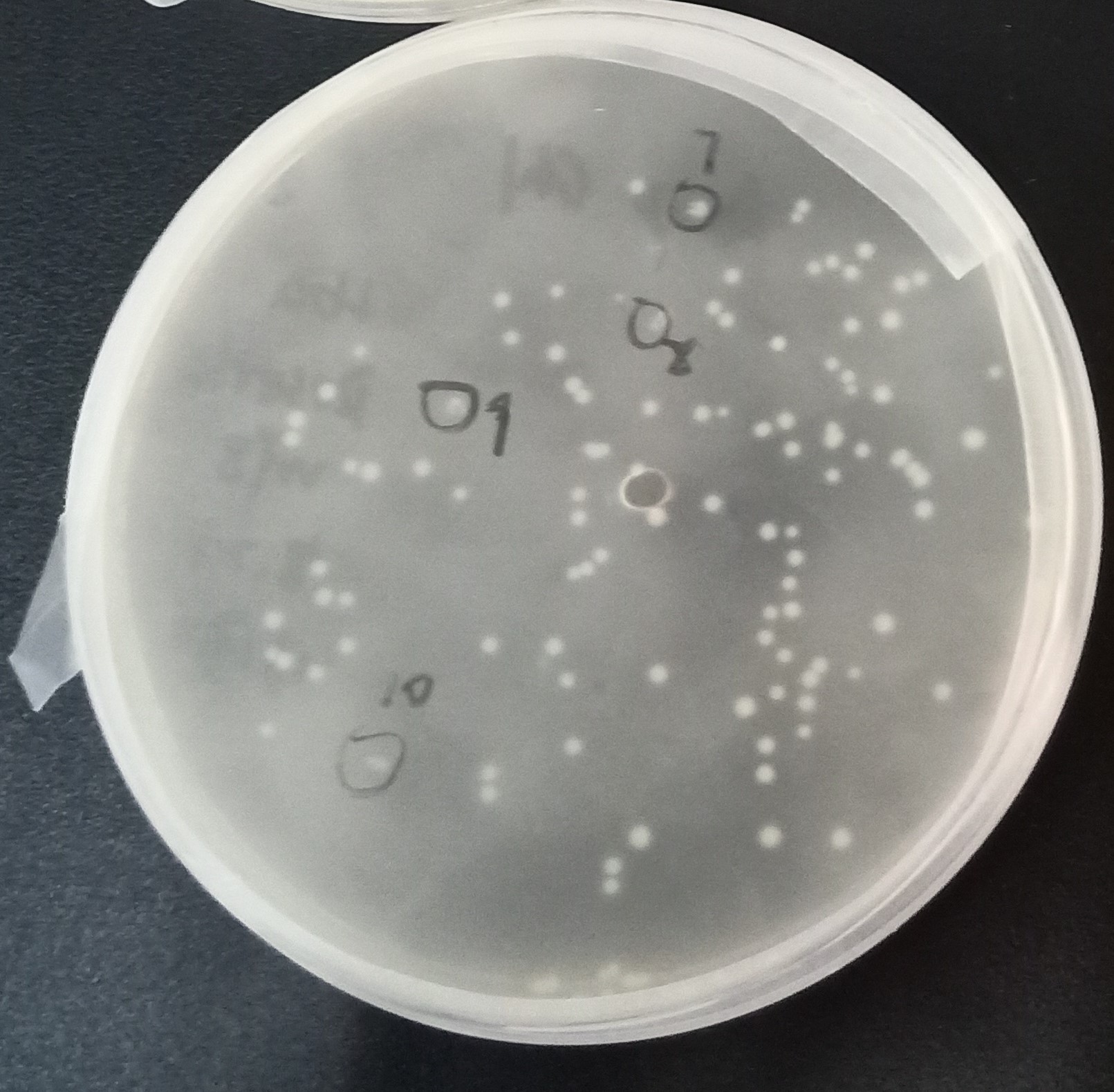
Figure 9. The transformants of recombinant plasmid His-SUMO-Cas13b-Cca
Result:
As shown in Figure 9, transformant colonies were found in Kanamycin resistant medium.
Identification of recombinant plasmid SUMO-CcaCas13b by colony PCR
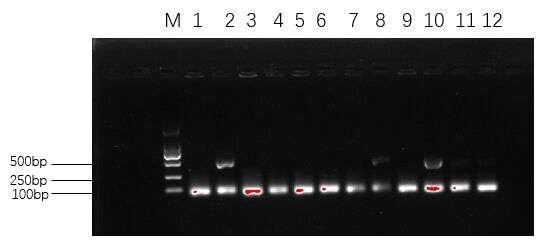
Figure 10. The agarose gel electrophoresis of His-SUMO-cas13b-Cca transformants.
Note:
Lane M:Marker 1kb plus
Lane 1 to Lane 12:His-SUMO-cas13b-Cca transformants
Result:
As shown in Figure 10, among the selected single colonies, only the second and tenth single colonies had bright bands in the range of 250 to 500 bp, which demonstrated that the recombinant plasmid was successfully transformed.
Cas13 protein homologues expression and purification
PsmCas13b
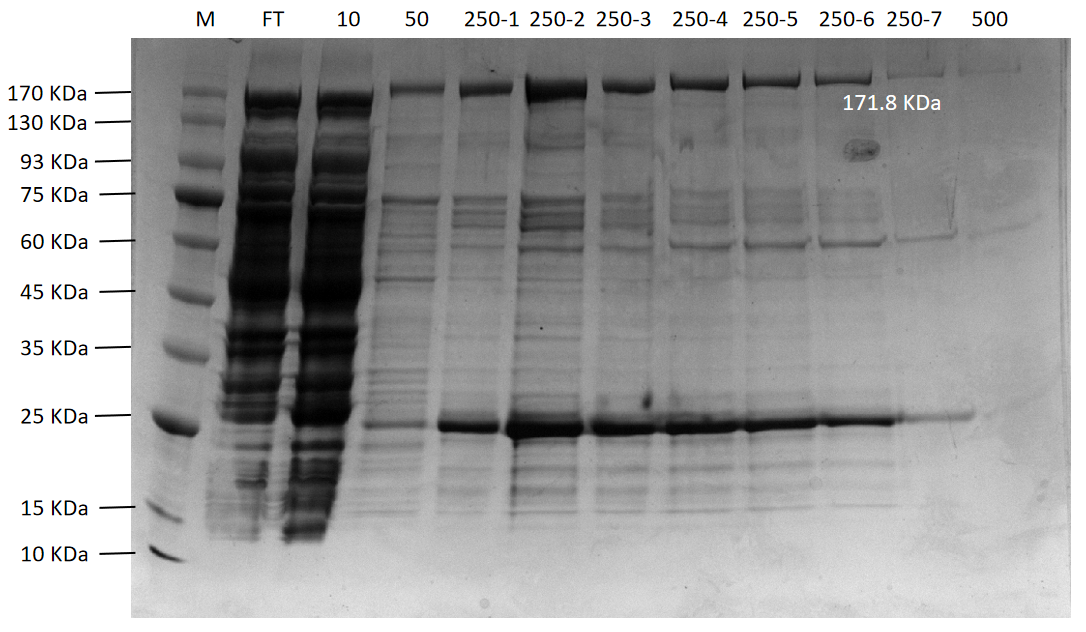
Figure 11. The agarose gel electrophoresis of PsmCas13b purification expression
Lane M:Protein Marker
Lane FT:Flow through liquid. After the cell lysate was incubated with Ni-NTA agarose
Lane 10:10 mM imidazole eluted protein flow through
Lane 50:50 mM imidazole eluted protein flow through
Lane 250-1 to 250-7:250 mM imidazole first to seventh eluted protein flow-through
Lane 500:500 mM imidazole eluted protein flow through
LbaCas13a

Figure 12. The agarose gel electrophoresis of LbaCas13a purification expression
Lane M:Protein Marker
Lane FT:Flow through liquid. After the cell lysate was incubated with Ni-NTA agarose
Lane 10:10 mM imidazole eluted protein flow through
Lane 50:50 mM imidazole eluted protein flow through
Lane 250-1 to 250-5:250 mM imidazole first to fifth eluted protein flow-through
Lane 500:500 mM imidazole eluted protein flow through
CcaCas13b
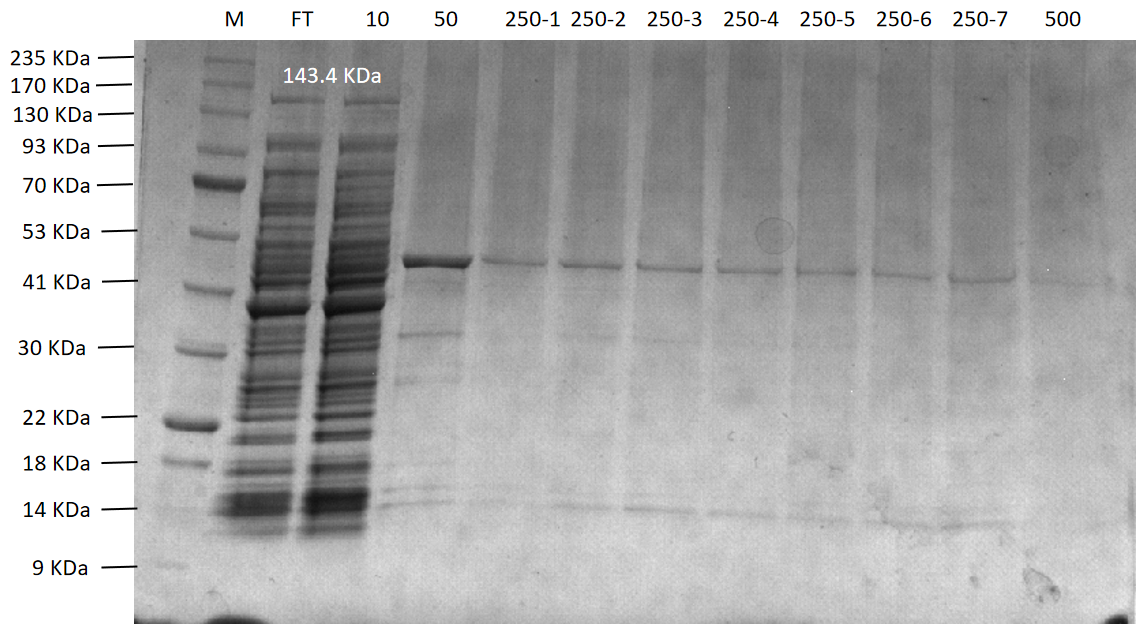
Figure 13. The agarose gel electrophoresis of CcaCas13b purification expression
Lane M:Protein Marker
Lane FT:Flow through liquid. After the cell lysate was incubated with Ni-NTA agarose
Lane 10:10 mM imidazole eluted protein flow through
Lane 50:50 mM imidazole eluted protein flow through
Lane 250-1 to 250-7:250 mM imidazole first to seventh eluted protein flow-through
Lane 500:500 mM imidazole eluted protein flow through
TDPs-33020
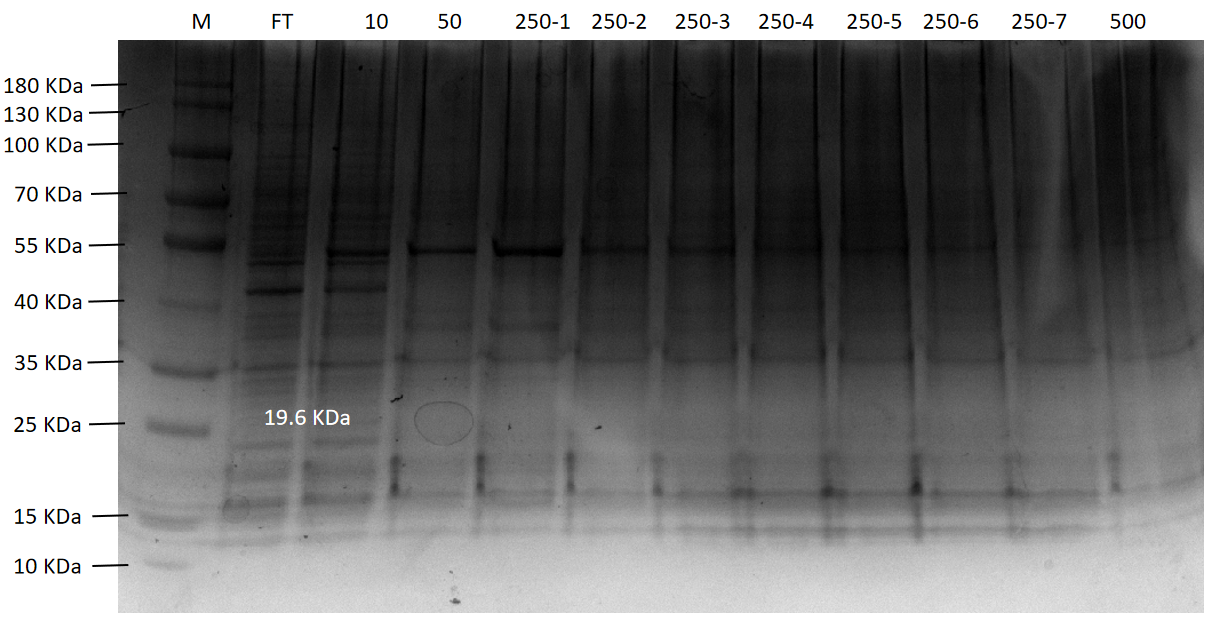
Figure 14. The agarose gel electrophoresis of TDPs-33020 purification expression
Lane M:Protein Marker
Lane FT:Flow through liquid. After the cell lysate was incubated with Ni-NTA agarose
Lane 10:10 mM imidazole eluted protein flow through
Lane 50:50 mM imidazole eluted protein flow through
Lane 250-1 to 250-7:250 mM imidazole first to seventh eluted protein flow-through
Lane 500:500 mM imidazole eluted protein flow through
Result:
Final purification results of four proteins (PsmCas13b, LbaCas13a, CcaCas13b, and TDPs-33020) were shown in Figure 11 to Figure 14, respectively. Among them, PsmCas13b and LbaCas13b are not completely pure proteins, but they are functional and can be used for subsequent fluorescence detection. In addition, CcaCas13b and TDPs-33020 have not been purified to the functional proteins. The proteins were purified with Ni-NTA agarose following the experimental procedures in the literature. Due to the problems of phages and purification equipment, we spent a lot of time o n it in the early stages.
Fluorescence detecting
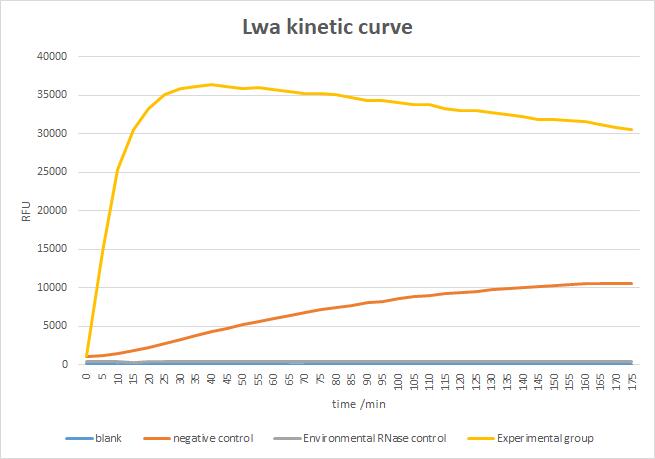
Figure 15. Cleavage activity of LwaCas13a protein

Figure 16. The cleavage activity of LbaCas13a protein
Result:
As shown in Figure 16, the activity of LbaCas13a protein is very low. Meanwhile, it seems that the activity of the expressed LbaCas13a protein was independent of the presence or absence of the target sequence. We speculate that the reason is that the soluble tag was not removed. Because the MBP soluble tag is very large, and it may be in the active center of the protein, which may affect the activity of the protein. Moreover, the inaccuracy of protein concentration data may also lead to the low activity of LbaCas13a protein.
We tested the detection limit of LwaCas13a protein (Figure 17). As shown in Figure 17, the LwaCas13a has a sensitivity of 10^- 13, which is quite sensitive.
Detection limit of LwaCas13a protein
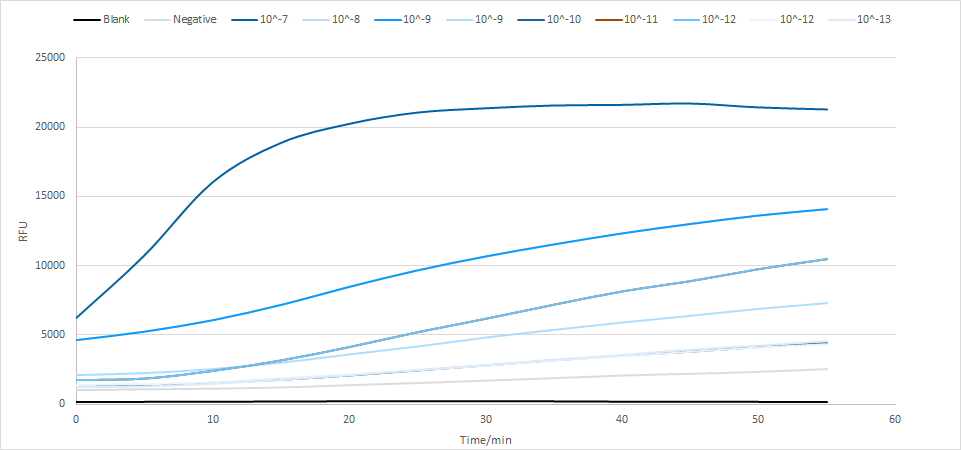
Figure 17. The limitation of LwaCas13a protein
Lyophilization
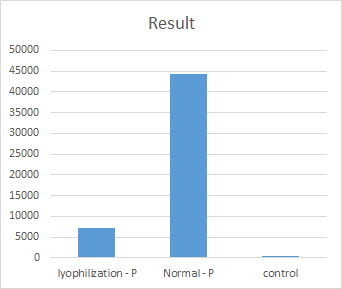
Figure 18. The cleavage activity of original and lyophilized LwaCas13a protein
Result:
The effect of lyophilization on the LwaCas13a was investigated (Figure 18). As shown in Figure 18, lyophilization can cause a notable loss of LwaCas13a. This will deeply influence the results. Therefore, the protein lyophilization should be optimized. To reduce the influence of lyophilization on protein activity, we plan to add tardigrade intrinsically disordered proteins (TDPs)to lyophilized detecting mix. However, due to the limited time, we did not complete this part before the deadline, so we listed it in our future steps.
Testing waste recovery
Our project aims to achieve simultaneous detection of multiple viruses. In order to realize our idea, we designed an efficient portable virus detection device based on CRISPR/Cas13 technology that can detect multiple viruses simultaneously.
The device is divided into the reactor part and the detector part. Since the biochemical reagents are sealed in both parts, we plan to recover the used reactor part, disposable gloves and swabs to ensure that the product will not be littered or abused after use. These issues are related to the recycling of medical waste. Since our knowledge in this area is limited, we interviewed the person in charge of Hangzhou Ecology and Environment Bureau to make our project more perfect and safe. Before the investigation and interview, we explained that the project device had not been put into actual production, and the research content was only for communication and learning.
1.Through the interview, we learned about the importance of medical waste recycling. The medical waste can expose medical staff, testers, waste handlers, patients and entire communities to infection and injury, as well as the risk of environmental pollution. Therefore, there are strict qualification requirements for medical waste recycling institutions, which include capital, technology, location, equipments and other requirements.
2.Since domestic medical devices have not been widely used, we learn that there is no domestic medical waste recycling system and relevant policies/regulations in China, which is a blind spot that can be easily ignored. Meanwhile, we also need to arouse people's awareness of the importance of medical waste disposal and follow the correct treatment methods.
3.Respondents advised us to contact a professional medical waste disposal company to solve the recycling and disposal problems. Medical waste recycling begins with the packing, labeling, and storage of the wastes by medical treatment units. Then the wastes were collected from temporary storage centers and transported to the qualified treatment centers. The waste was further treated in the centers.
4.In view of the low toxicity of biochemical reagents in our device to human body and the low content of samples in the reactor part, the interviewees indicated that the waste could be treated as general waste by high-temperature disinfection.
Finally, we learned that the following issues should be paid attention to when recycling waste from devices like ours:
1.Do not take it directly by hand;
2.Strictly mark warning signs and seal them in time;
3.Conduct chemical disinfection treatment or multi-layer package and then collect and treat as infectious waste;
4.The waste recovery and transportation process must be managed by a unified and professional enterprise/government agency.
|

