Project Design:
Introduction:
Spinal cord injury (SCI) is a devastating condition that alters every aspect of an individual’s life. Every year, 250,000-500,000 people worldwide suffer from SCI. In the UK approximately 2,500-3,500 people are diagnosed with SCI every year, with a total of 50,000 people living with it. As a consequence of SCI, over 2.5 million people worldwide live with paralysis (Patek and Stewart, 2020). Beyond its physical symptoms, SCI affects one’s mental and financial wellbeing, the impacts of which may extend to the patient's family and friends.
Due to the complexity of the central nervous system (CNS) and the elusive nature of its regenerative abilities, much remains unknown about SCI and how to approach it therapeutically. Despite the severity of this condition, there are few successful treatments (see “Current Treatments for SCI”) and this is precisely the gap which we aim to fill. At their core, the problems associated with SCI are due to the physical damage of the ascending and descending spinal tracts (a tract is a group of axons within the CNS with the same origin and destination; ascending tracts are somatosensory while descending tracts are motor). Following cell death caused by the initial injury, the axons in different neuronal tracts, that normally act as a highway, can no longer connect to each other, leading to a loss of motor and somatosensory function (Harrow-Mortelliti et al., 2020). Thus, to achieve functional restoration it is necessary to bridge the gap between the severed axonal pathways.
Biomaterial approaches have proven promising as a solution for SCI due to their capabilities in promoting axonal regrowth in the severed spinal cord region. The aim of our project, Renervate, is to develop novel biomaterial scaffolding that employs a mussel-foot protein (MFP)-based bioadhesive coating to encourage axonal regrowth and axonal guidance. The process of designing this scaffolding was complex, and required a thorough understanding of the anatomy of the spinal cord, the biochemical properties of spinal cord injury, and what would be needed from our scaffolding in a chemical, topographical, and mechanical sense for it to be a viable therapeutic option. These will be discussed in depth below.
Spinal Cord Anatomy:
In order to fully characterise our scaffolding, it was essential to understand the gross anatomy of the spinal cord. We visited the dissection lab in King’s College London, to visualise where we would insert our scaffolding and research into the properties we would need to mimic in our biomimetic scaffold. We were able to see the spinal nerve rootlets arising from each side of the spinal cord in pairs at the level of C1 to L1/L2, below which they formed a bundle called the “cauda equina”. We were mainly able to see the most external membrane, the dura, out of the three spinal membranes that surround the spinal cord: pia, arachnoid and dura. The dura loosely surrounds the spinal cord, which gave us a greater appreciation on how to cut into the dura in order to place our scaffold (Nógrádi and Vrbová, 2013).
The spinal cord is composed both of grey and white matter. If examined through a cross-section, the grey matter can be identified as the butterfly-shaped central part; it consists of cell bodies of interneurons, motor neurons and neutrophils. The white matter, which surrounds the grey matter, is mostly composed of axons as well as containing pathways that connect the brain with the rest of the body (Harrow-Mortelliti et al., 2020).

Neural pathways can be primarily distinguished between ascending tracts, which carry information from the sensory receptors to higher levels of the CNS and descending tracts, which carry info from the CNS to the periphery. An example of an ascending tract is the spinothalamic tract, which is responsible for carrying the sensation of pain, temperature, light touch and pressure from peripheral body parts. As aforementioned, the spinothalamic tract is an afferent sensory pathway and has no motor function. After a spinal cord injury, sensation is affected below the site of the lesion - damage to the spinothalamic tract results in contralateral loss of pain and temperature as the axons of this tract are severed (Harrow-Mortelliti et al., 2020; Patek and Stewart, 2020).

The spinal column can be divided into five regions - cervical, thoracic, lumbar, sacral and coccygeal. The cervical region consists of the C1-C7 vertebrae and is typically the most mobile region of the spinal column. It is followed by the thoracic region, which starts below the cervical region and has 12 vertebrae (T1-T2) - these function to support the neck and anchor the ribcage. The lumbar region (L1-L5) has the largest vertebrae compared to the other regions of the spinal column and they serve to support the upper body. The sacral region (sacrum) is made up of five vertebrae (S1-S5) that are fused together to form a single, triangular shaped bone. At the base of the spine is the coccyx, commonly known as the tailbone. Along with the sacrum, it helps support the weight of the body (Kaiser et al., 2020).
Injury to the cervical region of the spinal column accounts for 60% of SCI and is typically the most severe, as the cervical portion of the spine is closer to the brain and affects a larger portion of the body than the other sections. Cervical spinal cord injury is likely to result in either tetraplegia or quadriplegia, causing limited or absent sensory or motion functions below the shoulder/neck (Patek and Stewart, 2020). Depending on the site of the lesion, a cervical spinal cord injury can be more or less severe, with high cervical spinal cord injury often being deadly. Thus, our aim became to try and prevent the debilitating side-effects of cervical spinal cord injuries using our scaffolding.
Characterising SCI:
The pathophysiology of SCI is discussed in great detail on our Pathophysiology of SCI page.
Overview of SCI:
A spinal cord injury is characterised by damage to the spinal cord or nerves at the end of the spinal canal, which causes temporary or permanent changes in its function. SCIs can be classified as either complete - involving full loss of somatosensory and motor function - or incomplete - involving only partial loss of function (Alizadeh et al., 2019). Associated symptoms include loss of autonomic function, such as loss of bowel or bladder control, and chronic pain due to damage to the nerve fibres in the spinal cord (Hagen, 2015).
Spinal cord injury can be divided into three phases - acute, sub-acute, and chronic. Acute phase begins when the spinal cord is lacerated, contused or compressed by a force (in traumatic SCI) or obstructed by a vascular insult (in non-traumatic SCI), and encompasses the events of the immediate neurological damage. The mechanical injury leads to a cascade of biological events, which characterise the sub-acute phase of SCI. This occurs between minutes to weeks after the initial injury and leads to further tissue destruction. The subacute phase is typically defined by vascular changes (i.e. haemorrhage, breakdown of blood brain barrier and infiltration of inflammatory cells among others), free radical formation and lipid peroxidation, disruption of ionic balance, glutamate excitotoxicity, and apoptosis of neural cells (Alizadeh et al., 2019). The chronic phase, which can occur days to years after the initial injury, may lead to neurological impairments and compromising events, such as white matter demyelination, grey matter dissolution, connective tissue deposition and reactive gliosis. During the reactive gliosis, astrocytes and microglia proliferate and become reactive, together leading to the glial scar formation. In the glial scar, CNS glial cells, specifically astrocytes, create a cavity to seal off the site of injury epicentre and release chemo-repellents (Silva et al., 2014). (please see “Biochemistry & Microenvironment” for more information).
Table 1: The physiological and biochemical differences between healthy and injured spinal cord. In designing our scaffolding it was essential that we take into account all of these fundamental differences (Hausmann, 2003; Popovich and Jones, 2003).
| Healthy Spinal Cord | Injured Spinal Cord |
|---|---|
| Clear distinction between grey and white matter. | Grey and white matter destroyed and loss of distinctive boundary between them. |
| Intact blood brain barrier. | Breakdown of blood brain barrier. |
| Ionic homeostasis. | Loss of ionic homeostasis. |
| Absence of glial scar. | Formation of glial scar. |
| No inflammation, oedema, lipid peroxidation or oxidative stress. | Presence of oedema, inflammatory responses and lipid peroxidation. |
| No free-radical mediated damage. | Free-radical mediated damage. |
| Small scale production of nitric oxide. | Elevated production of nitric oxide. |
Based on this research, it was essential that we target a specific phase in SCI to design our therapy with specificity. As we started to develop our scaffolding design, we sought the help of experts to help clarify this point. We were advised by Dr. Zin Khaing that our solution should target the onset of the chronic phase due to in-patient processing and logistics of our scaffolding - as a consequence of this feedback we deemed it necessary to further our research into the chronic phase. Firstly, many SCI patients presenting polytrauma require treatment prior to open spine surgery. Secondly, patients may also need additional time to decide whether they would like to take on the risk of the experimental treatment and may hesitate to rush into it immediately after the initial trauma.
Pathophysiology of SCI:
Physical Changes During SCI:
After performing an extensive literature review on the anatomy of the spinal column and performing a spinal cord dissection at the KCL Medical School Dissection Lab, it was crucial for us to gain a comprehensive understanding of the physical and biochemical changes following the initial injury.
A trauma to the spinal cord involves the application of a variety of loads and stresses, leading to significant (temporary) geometric changes, which overcomes the tissue’s ability to adapt. Mechanical deformation can be the cause of functional and anatomical deficiency of spinal axons; injuries caused by mechanical deformation can result from compression, torsion or tension of the spinal cord. Compression results in a series of other biophysical events, such as permanent tissue deformation, which contributes to debilitating sensorimotor deficits (Alizadeh et al., 2019; Ouyang et al., 2010). By contrast, axotomy - also known as complete severing of an axon - causes immediate cell death by necrosis due to loss of neuronal membrane integrity (Hassannejad et al., 2018).
Biochemistry & Microenvironment of SCI:
The imbalance of the microenvironment of the spinal cord post SCI impairs both functional recovery and regeneration. Therefore, the aim of our scaffolding is to provide an alternative microenvironment that encourages axonal regrowth and restores function. Research into the biochemical and physical properties of the cyst (induced by SCI) allowed us to make an informed decision into which approach we would like to take during the implementation of our scaffold. Additionally, we conducted extensive and detailed research into the microenvironment of both a healthy and injured spinal cord - and associated biochemical pathways - to ensure our scaffold is as biocompatible as possible and non-immunogenic.
Mechanical damage to the local capillaries and severing of axons (known as axotomy), induces bleeding into the parenchyma of the spinal cord. This leads to reactive gliosis - the release of cytokines and chemokines from macrophages into the extracellular space is massively upregulated, causing damage to the blood-spinal cord barrier and increasing its permeability. The accumulation of macrophages in the microenvironment further promotes expression of cytokines and chemokines, continuing the depletion of the barrier and inducing inflammation. Wallerian degeneration - defined as active degeneration of the axons distal to the damaged neurons - accelerates the breakdown of cellular cytoskeleton, myelin clearance and inflammation. Inflammation, therefore, has a dual effect: it helps tissue remodelling and clear debris of lesion, but it also creates more cytokines and other molecules that promote further tissue breakdown (Fan et al., 2018). Due to the fact that inflammation can exacerbate mechanical damage to the tissue, it is crucial to investigate its effect on the microenvironment of SCI.
Mechanical damage to the spinal cord can also lead to a neural tissue edema, which may raise interstitial pressure and compress the surrounding vessels. This opposes the microenvironment of a healthy spinal cord where there is consistent blood perfusion, extensive vascular system, and a low number of inflammatory cells. This would normally prevent scar formation and eliminate any risk of oedema, lipid peroxidation and oxidative stress. In the healthy spinal cord, ionic homeostasis and no free radical mediated damage ensures the protection of mitochondria membranes and the correct function of the transmembrane adenosine triphosphate-driven pump (Nógrádi et al., 2006).
SCI results in neuronal death by both necrosis and apoptosis. Necrosis occurs as a direct result of injury due to damage to neuronal cell bodies, whilst apoptosis - programmed cell death mechanism - can be caused by multiple factors including DNA damage, hypoxia, cellular stress and inflammation (Zhang et al., 2012). During hypoxia, extracellular calcium ions move inside the cells, activating calcium-dependent proteases; this creates oxygen reactive species, leading to apoptosis. Cellular stress involves formation of oxygen free radicals and glutamatergic excitotoxicity - it can result from physical tear of the tissue, prompting cells to release their contents and therefore increasing neurotransmitter concentration in the environment; this means that cells keep having glutamate-powered bursts of activity, leading to toxicity. Excessive activation of glutamate receptors by excitatory amino acids leads to a number of deleterious consequences, including impairment of calcium buffering, further generation of free radicals, activation of the mitochondrial permeability transition and secondary excitotoxicity. Various types of cellular stress and exhaustion can lead to chromatolysis - an induced dissolution of the rough endoplasmic reticulum granules (known as Nissl bodies) - that occurs after cellular swelling and leads to neuronal death (Hausmann, 2003; Moon, 2018).

The secondary phase of the spinal cord injury involves the formation of the glial scar, which sets both physical and chemical barriers to neuronal regeneration. The scar contains reactive astrocytes, microglia, fibroblasts, and Schwann cells. It has been identified as a major inhibitory factor to neuronal regeneration after traumatic injury to the CNS. Moreover, fluid-filled cysts may also form at the site of injury and progressively expand, further contributing to cell death and axonal inhibition (Bradbury and Burnside, 2019). To help clarify the role of the cyst in the progression of SCI, we reached out to Dr. Zin Khaing, who advised that the cyst can expand up to 20% due to hypo perfusion and in order to achieve interaction between the cyst and our treatment, we would need to consider resecting the scar because the cyst is located outside of the functional spinal cord.
Regeneration in the Spinal Cord:
The barriers to endogenous regeneration within the spinal cord are its inhibitory environment and the reduced intrinsic ability of CNS neurons to grow. The inhibitory environment is caused by the natural repair mechanisms of the central nervous system becoming ineffective due to the imbalance of the inhibitory and excitatory factors implicated in neuroregeneration. Neurons cannot grow well through empty spaces, so the cut endings need to be stabilised before they can establish functional synapses (Huebner and Strittmatter, 2009). In the peripheral nervous system, the neurons are guided by schwann cells bridging the gap between the proximal and distal stump, forming “Bands of Büngner'', which guide neurons through an empty space. Schwann cells are also crucial in breaking and engulfing inhibitory myelin debris - when myelin is not cleared, macrophages continue to infiltrate the tissue, exacerbating chronic inflammation and preventing axon regeneration. Schwann cells are able to secrete growth permissive molecules, cell surface adhesion molecules and neutrophins and macrophages to promote neuronal survival (Jessen and Mirsky, 2016). By contrast, CNS glia - oligodendrocytes, astrocytes and microglia - do not express growth factors in temporal or spatial gradients supportive of regeneration. Instead, they express or secrete growth inhibitory molecules. Glial scar formation around the lesion sets both physical and chemical barriers to regeneration, which are further exacerbated by the inflammatory cell invasion. Notably, the glial scar releases the growth inhibitory matrix component chondroitin sulfate proteoglycan (CSPG). Overall, regrowth is hindered by the inhibitory environment, the formation of the glial scar and the reduced intrinsic ability of neurons to grow (Silver et al., 2015).
This led our team towards further investigation of the lesion’s microenvironment and how we could maximise conditions to mimic natural cell growth, promote axon guidance and provide structural support of the spinal cord. We initially discussed the use of growth factors such as HSPGs (heparan sulfate proteoglycans) in order to outcompete the interactions between CSPGs and their receptor. This interaction prevents axonal growth cone movement, thus inhibiting axons from passing through the glial scar and reduces the regenerative capacity of the axons (Tran et al., 2020). The limiting factor of this protocol involved the axons regenerating within the scaffold and being unable to propagate towards our target location, given that the growth factors provided an ideal environment.
This demonstrated the paramount solutions to axon regeneration as providing mechanical support and axon guidance for the cells to migrate. Since these neurons cannot grow effectively in empty space we decided on using a scaffolding, coated by a mussel-foot protein-based adhesive; they both promote axon regeneration through the scaffolding’s specific topography, topology and the mussel foot protein providing a surface for the spinal cord to adhere to, ultimately promoting cell proliferation and growth (Hoffman-Kim et al., 2010).
Current Treatments for SCI:
To design a more effective solution, it is necessary to understand pre-existing treatments and determine their faults. Presently, there is no established cure that would completely reverse the damage to the spinal cord. Instead, treatments often focus on preventing injury exacerbation and palliative care, aiming to rehabilitate patient's functional skills through occupational and physical therapy (Patek and Stewart, 2020). Due to the uniqueness of every SCI case based on its cause, location in the spinal cord and severity, none of the current therapy options can be applied universally or with the same success rate. Our scaffold directly targets this limitation as it is designed based on the patient’s MRI/CT scans in order to treat the primary injury.
Immobilisation:

Currently, the majority of treatments focus on minimising the damage to the spinal cord during the acute phase. Immediately after a SCI, the patient usually undergoes immobilisation, although it remains subject to the specifics of the injury. The need for immobilisation is assessed on scene by EMTs and is employed when there is a high index of suspicion for head or spinal injury . Altered mental states and neurological deficits are also indicators that this treatment must be used (Feller et al., 2020). Immobilisation maintains the anatomical integrity of the spinal cord, can realign the spine into its correct position if need be and prevents lateral movement of the vertebrae. It is widely used to ensure that there is no exacerbation of the primary injury, and can also reduce the severity of the secondary injury that follows. As immobilisation is done on-scene of the injury, patients can potentially be immobilised for several hours while they are transported to a hospital, meet with an emergency department physician, and then wait for a MRI/CT scan to be completed and read. Depending on location this process can take 2-3 hours. Prolonged immobilisation can lead to tissue ischemia, pressure ulcers and decreased lung volumes. Patients with a neck brace can also experience difficulty in managing their airways, potentially leading to an increased risk of pulmonary aspiration and a delay in tracheal intubation. The insertion of a central venous catheter can also be made more difficult (Ottosen et al., 2019).
Intravenous (IV) Methylprednisolone Sodium Succinate (MPSS):
Treatment during the acute phase can involve the administration of Intravenous (IV) Methylprednisolone Sodium Succinate (MPSS) (brand name Solu-Medrol). MPSS is a synthetic corticosteroid involved in upregulation of anti-inflammatory factors and decrease of oxidative stress. It enhances endogenous cell survival in animal models of SCI, reduces edema, prevents intracellular potassium depletion and inhibits peroxidation of lipids. Following the publication of the NASCIS II study, MPSS had become a standard of care when treating patients suffering from a SCI, but due to complications surrounding the use of the drug, its administration has become a highly debated topic among physicians and medical experts (Bowers et al., 2016). While it may seem that MPSS has a high efficacy considering the types of secondary injury associated with a SCI (inflammation of the lesion and surrounding tissue, free radical formation, decreased mitochondrial function etc), randomized controlled trials and observational studies do not support the use of methylprednisolone in acute SCI since it appears to have no long-term benefits. MPSS has been shown to cause an increase in gastrointestinal hemorrhage and other adverse events. Many regulating bodies in the neurosurgery field either strongly recommend, or advise against its use. Some professionals feel that the use of MPSS should be left to the attending physician, after they have balanced the risk of complication against the potential benefits on a patient-to-patient basis. In contrast to MPSS, our scaffold can circumvent the introduction of drugs that alter biochemical properties of the spinal cord microenvironment by solely focussing on achieving axonal regrowth using topography and topology (Ahuja et al., 2017; Rouanet et al., 2017).
Surgical Compression:
Surgical decompression remains a cornerstone of the acute treatment - it aims to realign the spinal column, re-establish spinal stability and decompress (i.e. relief of bony or ligamentous compression) of the spinal cord (Ahuja et al., 2017). It typically involves open reduction and decompression paired with an instrumented fusion (for example, using metal hardware) to stabilize the spine. The extent of surgery is tailored to the anatomical site, severity and extent of injury. The surgery is usually required due to progressive edema and hemorrhage that contribute to the ongoing mechanical pressure on the microvascular circulation - the surgery reduces secondary hypoxia and ischemia. Current recommendation is surgical decompression in the first 24 hours (Rahimi-Movaghar et al., 2009).
Palliative Care:
Palliative care for most SCI patients involves the inclusion of a wheelchair in their everyday life. Wheelchairs allow patients to move around both indoor and outdoor areas easily, ameliorating their quality of life through mobility. Typically, the severity of injury decides which wheelchair is most appropriate to the patient. These may vary between a manual or power wheelchair - manual wheelchairs are more affordable and portable but have limited adjustability, whilst power wheelchairs are customisable and easier to manoeuvre. However, they can be expensive, have a limited speed, and, in some cases, may require a van for transportation. SCI patients appear to be more prone to developing pressure sores as they cannot feel below the level of injury (Ekiz et al., 2014). This means that the wheelchair must be equipped with a pressure relief cushion in order to prevent further exacerbation of the injury (Fogelberg et al., 2009). Based on this problem, our scaffolding was designed to prevent the risk of a patient being placed in a wheelchair and instead focuses on re-establishing mobility through axon guidance and mechanical support.
Conclusions, Current Research, and Future Developments:
A therapeutic option similar to our proposed project - entitled INSPIRE - was developed by InVivo Therapeutics, with the last update of the clinical trials posted in December 2019. It involved implantation of a biodegradable Neuro-Spinal Scaffold into the epicenter of the post-irrigation contusion cavity during open spine surgery for treatment of thoracic acute SCI (Ahuja et al., 2017). It was assessed based on its safety and improvements in ASIA Impairment Scale grade, motor scores and sensory scores. Results have shown only 31% improvement in neurological level of injury (i.e. caudal change), 62.5% improvement in Sensory Pinprick Scores after 6 months, 62.5% improvement in Sensory Light Touch Scores after 6 months, and a mere 6.7% improvement in Total Motor Scores after 6 months (with no change for the rest of 93.3% participants). The spinal cord adhesion to the scaffolding was achieved in only 1 out of 16 participants, with the benefit of the treatment therefore being described as “probable” but uncertain (Theodore et al., 2016). However, this solution targets thoracic acute SCI and has a small participant group, making it difficult to compare it to our project. We aim to greatly improve spinal cord adhesion by incorporating mussel-foot protein based bioadhesive coating.
Both hydrogels and biomimetic scaffoldings serve as novel therapies for spinal cord injuries. Hydrogels are primarily designed to target the acute phase of spinal cord injuries, whilst scaffoldings are more successful in the treatment of the chronic phase. This distinction informed our decision on which therapy was most suitable for our project design. Although hydrogels can easily include a drug delivery system, they have a limited scope as they do not embody complex design strategies (Billiet et al., 2012). Relative to our project design, this proved to be disadvantageous as we planned to create a personalised therapy, unlike the current standardised treatments on the market. Instead, scaffoldings require a fully characterised internal architecture, that matches the mechanical properties of the spine, which allows for a more personal treatment. However, biomimetic scaffoldings are also more invasive and some materials can degrade and release lactic acid within the cerebral spinal fluid (Wang et al., 2018). Therefore, we carefully studied the different candidates for our scaffold making sure to take this into account and ensure our scaffolding does not further aggravate the patient’s injury. Coupled with the ability to promote axon guidance through topography and the effectiveness of the scaffold in the chronic phase, our scaffolding had clear advantages over the hydrogel.
Evolution of Our Solution:
Our solution and project aims are discussed in greater detail on the Project Description page.

Our initial literature review pointed to a hydrogel-based solution for SCI repair. In particular, we sought to develop an injectable, protein-based hydrogel that, once injected into the lesion site, will expand to fill the area and provide a surface that supports axonal regrowth. A single injection makes the treatment less invasive as a second surgery is not required to remove an implant. Our protein candidate for the intended hydrogel was Pvfp-5, an isoform of a mussel-foot protein (MFP) that is derived from Perna viridis, the Asian green mussel. Based on the literature, it is evident that adhesion plays an essential role in ensuring axonal regrowth. The particular mussel-foot protein chosen is crucial in the adhesion of the mussel to surfaces, even in turbulent water. Additionally, it has been shown to be biocompatible, non-cytotoxic, and have strong mechanical properties. Upon encountering this protein through our research, we believed it would be ideal to include in our protein-based hydrogel and would be combined with other well-established materials. In doing so, we originally aimed to create a novel, bio-adhesive hydrogel that fulfils the essential requirement of adhesion in neural tissue engineering.
However, due to the Covid-19 pandemic we recognised that it would not be possible to access the lab. Thus, we made drastic changes to our project design. After revising our design, we decided upon a bioprinted scaffolding. This procedure was chosen for a plethora of reasons. Firstly, we realised that we could work towards our original aims remotely by 3D-printing a solid scaffolding structure that could be implanted rather than injected. This would allow us to continue to work towards the development of a solution for SCI even as our team is scattered across the world. In particular, we decided to carry out 3D bioprinting to continue our research into biomaterials. Secondly, we also found that previous solid scaffolding structures employed MFP’s to achieve adhesion – an issue that is not limited to hydrogel technology. Thus, we could continue to use Pvfp-5β as a component of our design and to ensure that we apply Synthetic Biology to our project. Rather than developing a material that directly depends on the protein, we decided to 3D bioprint a material that incorporates the protein. Thirdly, the scaffolding offers several advantages to a hydrogel particularly relating to SCI treatment, as discussed above. Thus, we redirected ourselves towards a biomaterial-based scaffolding that utilises our MFP to ensure adhesion.
Overview of Our Scaffold Design:
Hereunder is an overview of how we designed our scaffold. We go into much more depth regarding our research and thought process on the Scaffold Design page.
Scaffold requirements:
Through research, we generated a list of scaffold requirements which included: biodegradability, bioresorbability and biocompatibility. We determined our scaffold would need to have mechanical properties similar to that of the spinal cord and needs to be able to be personalised to fit the glial scar of each patient, having a shape that will assure the accomplishment of its objectives. Subsequent to the macro-architecture of the scaffold, the micro-architecture are crucial, requiring optimal pore sizes. We validated our scaffold requirements as part of our integrated human practises and from these validated scaffold requirements, we created a scaffold specification sheet. We ensured each engineering decision taken was in line with our scaffold specification sheet and hence our human practises.
Material Selection:
We shortlisted some candidates for our scaffold, these included: collagen, chitosan, PCL, PLA, PLGA and PCL composites, the latter four being synthetic polymers and the first couple being natural polymers. After evaluating these candidates, we decided that our best solution was a PCL-chitosan blend. This natural-synthetic blend was chosen due to the fact that the advantages of each type were combined. PCL has a high tensile strength, a high Young’s Modulus (0.21-0.44 GPa) is biodegradable and is biocompatible, as it elicits a minimal immune response. However, we wanted to include chitosan as it was renowned as one of the best and most effective drug delivery systems, and so we could have the benefits of the natural materials. Chitosan is able to interact with a wide variety of molecules, is biocompatible, biodegradable, as well as non-toxic. The scaffold combined with the drug delivery system would allow for axon regeneration alongside localised, controlled release of crucial drugs such as anti-inflammatory drugs, HSPGs and heparin-binding growth factors (FGF-2 and HB-EGF) to name but a few.
Novel therapeutics research has mainly focused on the delivery of pharmacological compounds, known as Drug Delivery System (DDS). Drugs can be delivered systematically, affecting the whole body, or locally. Local delivery results in better achievements, as it represents a way to decrease side effects of drug toxicity as well as maximising the effectiveness of the treatment. The development of a successful drug delivery nanosystems results in the scaffold having beneficial neuro-protective and neuro-regenerative effects. Due to the benefits DDS could provide to the injured site, it was one of the characteristics we looked into when choosing the right material for our scaffold. Whilst we were challenged with inaccessibility to the lab and time constraints to any possible laboratory access, we recognised the benefits of choosing a material for the scaffold that could act as a drug delivery system as an option for the future implementation of the scaffold.
Because of properties such as nontoxicity and biodegradability, PCL has been very popular in the field of drug delivery. Numerous drugs have already been incorporated in PCL scaffold, in the form of nanoparticles or nanofibers. Although, current fabrication of a 3D printed porous scaffold including drug delivery system has not been developed. Taking this into account, if we were to include a DDS into our project some drugs which we would particularly investigate would be melatonin and dexamethasone acetate. Melatonin is a versatile hormone with many properties such as antioxidative, antiapoptotic, neuroprotective, and anti-inflammatory. Different studies have suggested the restoration of neurologic functions after SCI, involving mechanisms such as antioxidant effects, regulation of iNOS, inhibition of proinflammatory cytokines, blood vessel repair, restoration of the blood-brain barrier, inhibition of cell apoptosis, edema resistance and inhibition of cell autophagy. As well as melatonin, dexamethasone acetate it’s known to have neuroprotective effects by inhibiting lipid peroxidation and inflammation due to the reduction of cytokine release and expression. However, it has some limitations caused by its hydrophobicity, biocompatibility and numerous side effects if using large dosage. After being advised by specialist Jacob Koffler, we were decided to maintain our project design as simple as possible; therefore, including a DDS into our scaffold would have only been a complication to the realization of the final product. Although, we are aware that without any GF or drug released, the realization of an environment advantageous for the regeneration of the axons, will be very hard to achieve.
Our research into DDS supported the use of PCL-Chitosan blend for the scaffold, however, after meeting with a specialist, Dr Riehle, he advised us against using chitosan stating that “chitosan is immunogenic and would have to be hidden.” The complications arising from this issue were stark, for example, we would have to include another material to surround the chitosan and prevent the body from detecting it. After much deliberation, we came to the agreement of using PCL as a standalone material for our scaffold. This decision was further validated with our degradation model.

Poly-ε-caprolactone (PCL) is a non-toxic (FDA approved), low-cost, bioresorbable polymer with excellent mechanical properties and a slow degradation rate. Moreover, it has a relatively low melting point of around 60°C, meaning that it is particularly suitable for 3D bioprinting. It has a compressive strength of 2.77 ± 0.26 MPa and takes > 24 months for complete degradation, which is an important feature because a scaffold must be biodegradable to allow cells to produce their own extracellular matrix. The mechanical properties and the degradation rate can be further manipulated by altering the molecular weight, such that it remains within the spine for an appropriate length of time - until regeneration is complete, without the need for further surgical procedures to remove it.
Macro- & Micro-architectures:
There are three primary reasons for using scaffolds in the treatment of SCI; 1) to reconstruct spinal cord tissue architecture, 2) provide guidance for regenerating axons, and 3) to prevent the infiltration of the scar tissue. An advancement of this is to engineer the micro and macro-architecture of the scaffold to amplify the aforementioned. The macro- & micro-architectures are discussed in much greater depth here.
Micro-architecture:
Micro-architecture describes the imposed topography on the material and how it can affect cells on the surface by further advancing adhesion, spreading and alignment of axons. One configuration is to apply microgrooves on the surface. A literature review has said that with micro-grooved topography, neurite response increases with increasing groove depth, starting from 0.2 – 4 μm. Increasing the groove height resulted in both increased alignment and restriction of neurites. Another technique is to control the pore size. A scaffold with pore size between 150-600 μm is in the accepted range for cell growth, migration, and vascularisation. Cell adhesion is vital, “the performance of nerve guidance channels has been further enhanced with more advanced designs that focus on cell adhesion”, this emphasises the need for cell adhesion. Specific adhesion is when channels that are modified with proteins derived from the extracellular matrix (ECM) will closely mimic the environment, meaning greater regenerative success. Lastly, dense PCL does not exhibit desired properties such as porosity, stiffness, strength, and cell adhesion to function as a scaffold; creating controlled porosity conditions will solve this. By modulating porosity, the elastic modulus can be adjusted to ~ 2.09 -182 MPa - this is crucial because the mechanical properties of the scaffold should be as closely matched to the spine as possible. Along with this, scaffolds that were produced with 60% open volume were hypothesised that functional nerve repair would be achieved compared to the current standard of 45% open volume. We have also considered using a micro/nanostructure film on the surface of the scaffold. Specifically, the nano-pillar projections utilised in gecko feet, which have shown to significantly increase adhesion when used alongside DOPA containing polymers, which would provide greater stability for our scaffold in the SCI site. This is especially useful as stability is needed in the fragile SCI environment, alongside the fact that PCL and other synthetic polymers do not adhere strongly alone.
Moreover, due to not incorporating a drug delivery system, alternative ways of addressing the issues presented by SCI must be considered. For instance, literature has hypothesised that the size and shape of pores may affect macrophage behaviour and cytokine secretion; cell differentiation can depend on the substrate’s geometry, and hence negative inflammatory responses could be mitigated by considering such. Further to this, it was shown that cells may adopt a more elongated morphology when adhered to smaller pores of tighter angles. Conclusively, carefully considering the micro-architecture of the scaffold not only benefits the axonal growth, cell adhesion and mechanical properties, but may be able to aid in solving some of the issues that would otherwise be addressed via drug delivery.
Macro-architecture:
Alongside the micro-architecture, the macro-architecture plays a crucial role in the effectiveness of a scaffold. From the literature we have read, the most effective structure was the open path with a core. This was designed to support the white matter tracts from the grey matter in the centre. Additionally, it allowed for the extension of myelinated fibres along the length of the injury both exterior and inside the scaffold. Furthermore, axon regeneration was observed in this design. The open path with core design marginally outperformed the open path without core design. Theoretically, the core could provide a surface for guiding white matter tracts. The other macro-architectures discussed were open path without a core, cylinder, tube and channel implants, all of which had the defect double in size and with no neural tissue bridging.
A further important characteristic of scaffold design is its innate ability for cellular adhesion; a scaffold should facilitate three-dimensional growth of cells and axons in an organised manner. Properties such as chemistry and topography can alter this greatly, and so it is important that an extensive range of literature is considered to optimise these. The surface of the scaffold is the closest site of interaction with surrounding cells, and as a result, it should be able to enable the attachment of such. Within tissue engineering, it is imperative that the scaffolding is as similar to the in vivo environment as possible to encourage axons to traverse the ‘bridge’ across the injury site and encourage a high-density cell population to aid repair.
The most favourable method of improving cell attachment, based solely on design, is increasing the surface-area-to-volume ratio so that the ideal number of cells to restore function can be anchored – this may be achieved by introducing sufficiently small micro-pores, and a ‘rougher’ surface. However, PCL, despite having a range of positive features such as elasticity, biodegradability and drug permeability, has poor cellular adhesion properties – similarly to other synthetic polymers. The prime reason for this being its hydrophobicity, reducing cell interaction. Due to its popularity in tissue engineering, the minimisation of this issue has been investigated by many research teams; with some solutions consisting of plasma treatment, coating the scaffold (with proteins such as collagen), or creating a hybrid polymer (a blend of natural, synthetic polymers or bioceramics).
Plasma treatment, albeit not having a detrimental effect on the mechanical properties of PCL and little inflammatory response, has evidence suggesting that its added benefits are not as effective in the long-term. Conversely, according to Dr. Mathis Riehle, the recommended type of collagen for scaffold use in the CNS is III and IV – which is problematic due to cost and the source of the protein: often, the collagen is taken from mice tumours and so is not suitable for in vivo use. Finally, the creation of a hybrid polymer may prove to be challenging – especially without lab-access – due to the need for testing different blends to obtain the perfect ratio of each material, and because the availability of prefabricated blended bioinks is scarce. Additionally, incorporating another material may lead to the compromise of the initially attractive properties of PCL; for example, along with the aforementioned issues with the utilisation of chitosan, it may also affect the control of the micro-architecture because of its tendency to expand in aqueous environments, and as a result the mechanical properties would no longer be ideal. Conclusively, there is still a need to find a viable solution to the poor adhesion of poly-ε-caprolactone; we think that our novel approach of including Pvfp-5β is a good candidate to fix this. After a meeting with Dr. Riehle, we found that there are several scaffold coating methods. After our discussion, we decided the most appropriate method would be to submerge the scaffold into our MFP based adhesive. This would ensure the micro-channels are filled with MFP, helping direct axon proliferation by providing a surface for adhesion.
Scaffold Bioprinting:
What is required of a successful scaffold for spinal cord injury treatment is discussed in great detail on the Scaffold Design page.
3D Bioprinting:
Conventional methods for fabricating scaffolds consist of, albeit are not limited to, three main categories: using porogens in biomaterials (e.g. solvent casting and particulate leaching), techniques involving fibres (such as electrospinning), and solid freeform fabrication. Each have given advantages and disadvantages; for example, utilising porogens allows the creation of a scaffold that can easily match the physical properties of native ECM, yet often results in low cell viability – and, as a result, sacrifices must be made in regard to physical properties (such as pore size) to mitigate this issue. Additive manufacturing (3D printing) is particularly attractive due to its capability of producing extremely repeatable designs with complex shape and porous structure, using a high resolution – which can also be matched, precisely, to a patient’s lesion. Unlike porogen-centred processes, bioprinting can produce precisely homogeneous micro-architecture; the distribution of pores must be exact to produce optimal regrowth. Moreover, 3D bioprinting minimises waste, due to its nature of creating a structure layer by layer, as opposed to subtractive methods.
Printing Method Selection:
An important consideration regarding the success of the scaffold is selecting the appropriate printing method; commonly used printers, predominantly inkjet, can negatively impact mechanical integrity due to planar artefacts being formed during fabrication (which is a crucial aspect of scaffold design). Moreover, compromises must be made between the time taken to print the scaffold, and the accuracy of the final print across different technologies; however, generally, solid freeform fabrication is a quick process – with some literature stating that a 2mm scaffold could be created within 1.6s using a μCPP method.
Determining the Proposed Implementation:
How will we 3D-bioprint the scaffold? What will the process look like after a patient suffers from a SCI? Firstly, CT or MRI scans will be performed on the patient. Using this data, we will then construct a scaffold design using AutoDesk Inventor (using this design model, it will be recreated layer-by-layer using PCL bioink obtained from a third-party source). Following this, the micro-architecture will be implemented using Rhinoceros. The model will be sliced using Cura software and is then transformed into a g-code file ready to be fed into a 3D-bioprinter, ready for printing. The purpose of the slicer software (Cura) is to give the bioprinter instructions, e.g. build temperature, extrusion pressure, and speed. The bioprinters we will use are high precision printers from the Dentistry department at King’s College London. This process allows us to create tailored scaffolds using ITK SNAP for each and every patient, as no two spinal cord injuries are identical. After the scaffold has been printed it will then be modified. This process involves submerging it in our mussel foot protein for adhesion.
Role of Adhesion:
Mussel foot proteins and their applications as bioadhesives are described further on our mussel foot proteins page.
Adhesion has been shown to be essential in guiding successful axon regeneration. Using a scaffold to bridge the gap produced by the SCI cyst, where axons have no attachment and cannot propagate, provides a partial solution to this issue. Many scaffold materials alone are not adhesive enough to support growing axons; by coating our scaffold in MFP, we can provide a consistent and reliable attachment point for axons throughout the scaffold. MFP’s have been shown to aid actin filament regeneration in immortal cell lines (HeLa/NIH-3T3), thereby providing a promising route into aiding axon propagation. We hope to utilise this property in guiding functional axon growth within our scaffold.
Why employ Pvfp-5β?
After evaluating a review paper on hydrogels, we discovered the rising potential in mussel foot proteins (MFP’s) for use as biological adhesives. The issue with current adhesives is that they do not maintain their adhesiveness in aqueous environments. Mussels have evolved a way to maintain adhesiveness underwater in the form of MFP’s, highlighting their potential to be used within the body for sutureless surgeries, as well as reducing the need for metal pins to secure structures in place. This would provide us with an effective and non-invasive way of implementing our scaffold into the CNS, essential to favour axon regeneration. MFP’s contain catechol groups in the form of DOPA residues (3,4-dihydroxyphenylalanine), an adhesive molecule with a wide range of different bonding possibilities, which enables the mussel to bind to a variety of different surfaces (Lu, et al. 2012). After researching into the properties of MFPs from a range of different species, we decided to look further into Pvfp-5β. This MFP is the first to be secreted into the mussel foot in the formation of the mussel byssus in the asian green mussel (Perna viridis). It contains a high percentage of DOPA in comparison to other MFP’s, and adopts an unordered and elongated structure which maximises DOPA interactions with the contacting surface (Petrone, L. et al. 2015). As the mussel byssus gradually evolved to withstand turbulent tidal forces which bombard the surfaces in which mussels attach, the protein has also adapted and enhanced its adhesiveness. We hope that the incredible adhesive ability of Pvfp-5β will ensure our adhesive is strong enough to keep our scaffold secure, especially as the spine is a very mobile part of the body. Many mussel foot proteins, including Pvfp-5β, have been successfully synthesised in recombinant E. coli, providing a confirmed route into synthesising our MFP (Hwang, et al. 2004)(Santonocito et al. 2019). We therefore identified this MFP as the ideal candidate for our adhesive.
After further investigations into Pvfp-5β, we found that MFP’s have been shown to elicit minimal immune responses within the body (Santonocito et al. 2019). The biocompatibility of MFP’s reduces the complications of the immune system damaging our scaffold, also reducing any unanticipated immunogenic complications. After contacting Dr. Koffler, we discovered that the scaffold must maintain structurally sound for at least 6 months to ensure suitable recovery has taken place for the body to continue to repair once the scaffold degrades. Coating our scaffold with a bioadhesive MFP polymer would thereby reduce this degradation via the immune system and negate the need for immunosuppressant therapy.
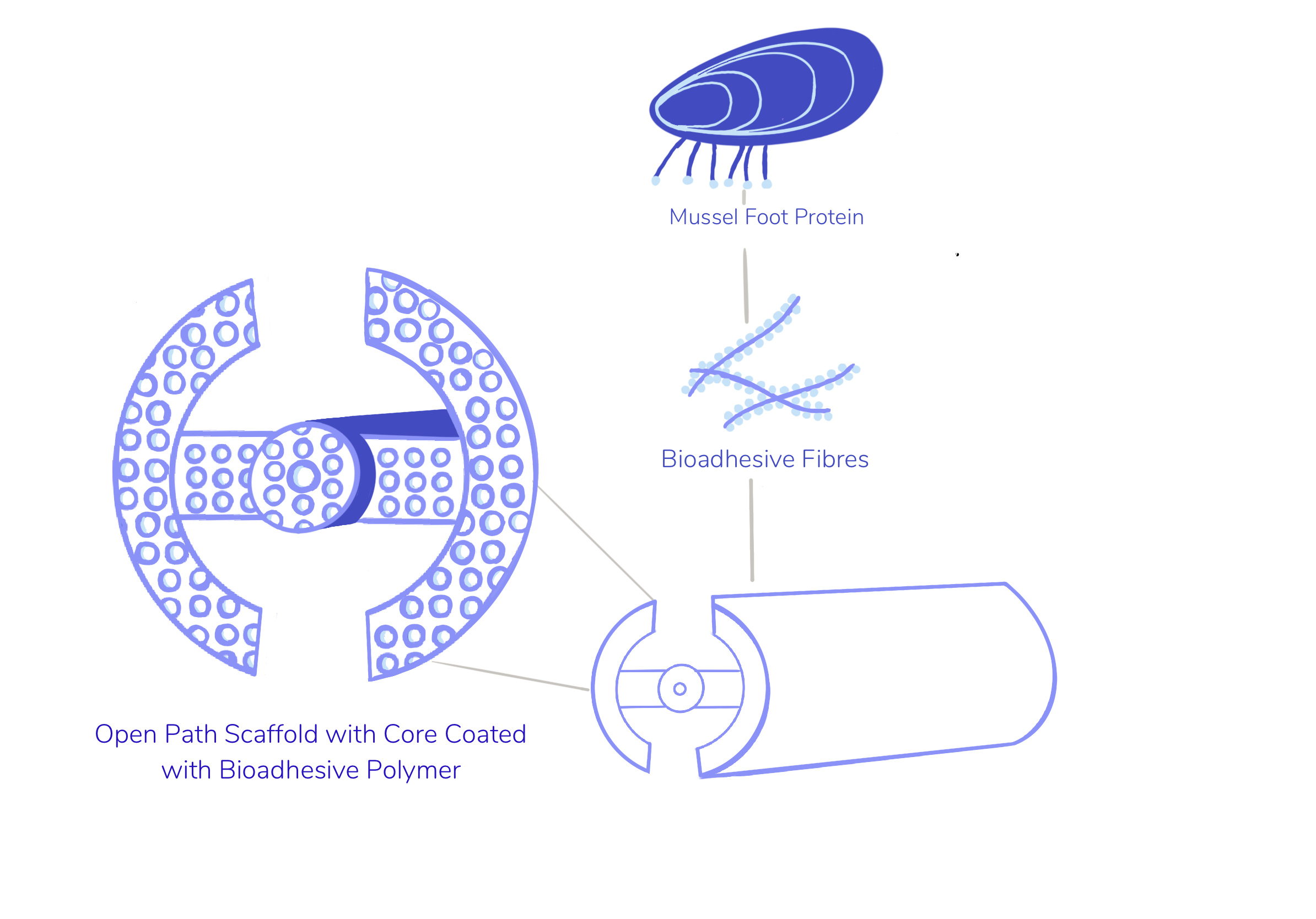
Creation of a Pvfp-5β-based Bioadhesive Coating:
Appropriate Application of MFP:
We believe we have found the optimal adhesive force we could produce in the synthesis of our adhesive. This would be achieved by using a mixture of Pvfp-5b and surface nanostructures to maximise the surface area. With further studies and experiments into mutagenesis, we hope to improve the adhesiveness of Pvfp-5b even further. However, after a meeting with Dr. Zin Khaing, we discovered that if Pvfp-5β is too adhesive, it could restrict axons propagating across our scaffold. Despite this, we feel having found the maximum adhesive force, that we can now adjust the application of our adhesive, balancing axon growth and support of our scaffold. We will explore this further during the the Phase II aspect of our project once we have lab access.
Prevention of Oxidation:
After researching into the adhesion mechanism of Pvfp-5β and MFP’s, we learned that DOPA residues are prone to autoxidising into DOPA-quinone in physiological conditions (pH-7.4). In nature, the mussel combats this by creating an acidic environment (pH 3.5-5) by secreting H+ ions into the mussel foot (Waite, H.J. 2017). From this, we identified that the increased pH within the body acted as a barrier in our project. In its oxidised form, DOPA is severely limited in its adhesive capabilities (Kan, et al. 2014). This is due to the loss of catechol hydroxyl groups responsible for bonding with the surface (see Figure 3). Preventing the oxidation of DOPA residues will result in the retention of its adhesive functionality, required to ensure the stability of our scaffold within the spine.
We have identified several biomimetic and synthetic approaches to this barrier, including the chlorination of the catechol groups (as seen in Sandcastle worms), the incorporation of MFP-6 (an MFP produced by the mussels which reduces DOPA-quinone back to DOPA via thiol groups) and the use of catechol protecting groups such as boronate, which can be employed to temporarily shield DOPA from oxidation. We spoke to Dr Petrone about this, to which he suggested chelating DOPA residues with ferric ions. After contacting him, we decided to further pursue the use of boronate complexes and iron-chelation as our main research focuses. Upon presenting our options to Professor Pastore and Dr Alfano, they advised us to follow an iron-chelation based solution to this issue; however, they flagged potential issues such as the precipitation of MFP and the potential oxidative effects of iron ions on cysteine residues, fundamental to MFP structure and antioxidant properties. This information guided us when conducting further research.
DOPA-iron complexes are resistant to the oxidative effects of high pHs, thereby providing a solution to our problem (Menyo, et al. 2013). Iron chelation is optimised between DOPA residues in the sclerotization process of the mussel byssus, alongside DOPA-quinone crosslinking. In increasingly basic conditions, DOPA favours the formation of bi- and tri-dentate coordination complexes thus providing an effective way to produce crosslinking between MFP’s (see Figure 8 )(Budisa, et al. 2019).

We also researched into incorporating MFP-6 (Pvfp-6) into our project design – an idea piqued by the redox capabilities of Fe3+ at high pHs (see Figure 3). MFP-6 is able to donate electrons to DOPA-quinone, thus reducing it back to DOPA and restoring its coordination complex mediated cross-linking and adhesive properties. This will also ensure that the oxidation of cysteine residues will not cause any unpredicted changes in protein structure, highlighting the importance of our protein model (Yu, et al. 2011)(Waite, 2017). We also hope this will combat any unexpected source of oxidation within the spine.
Process of Polymerisation:
p(DEA-co-MEA) (Poly(dopamine methacrylate-co-2-methoxyethyl acrylate)) is a DOPA containing polymer which utilises vinyl groups for polymerisation. This is a simple process involving a free-radical mediated reaction, easily stimulated with UV light via a photopolymerisation process (Glass, et al. 2010)(Haeshin, et al. 2004). These polymers could then be linked via crosslinking agents such as EGDMA (ethylene glycol dimethacrylate, a divinyl linking agent) or iron chelation. Dr. Riehle highlighted that making our scaffold too rigid may damage the SCI site further, as the spine is a flexible structure; part of the success of injection-based scaffolds. Iron chelation would provide more flexibility in our scaffold, as the coordination complexes formed between iron and DOPA are fully reversible. As these complexes are reversible, our adhesive would have self-healing properties, which we hope will resist any excessive motions within the spine which may lead to displacement of the scaffold, leading to further damage. As well as this, using this method would coincide with our previous iron chelation research.
References for SCI:
- Ahuja, C.S., Wilson, J.R., Nori, S., Kotter, M.R.N., Druschel, C., Curt, A., Fehlings, M.G., 2017. Traumatic spinal cord injury. Nat. Rev. Dis. Primer 3, 1–21. https://doi.org/10.1038/nrdp.2017.18
- Alizadeh, A., Dyck, S.M., Karimi-Abdolrezaee, S., 2019. Traumatic Spinal Cord Injury: An Overview of Pathophysiology, Models and Acute Injury Mechanisms. Front. Neurol. 10. https://doi.org/10.3389/fneur.2019.00282
- Billiet, T., Vandenhaute, M., Schelfhout, J., Van Vlierberghe, S., Dubruel, P., 2012. A review of trends and limitations in hydrogel-rapid prototyping for tissue engineering. Biomaterials 33, 6020–6041. https://doi.org/10.1016/j.biomaterials.2012.04.050
- Bradbury, E.J., Burnside, E.R., 2019. Moving beyond the glial scar for spinal cord repair. Nat. Commun. 10, 3879. https://doi.org/10.1038/s41467-019-11707-7
- Ekiz, T., Özbudak Demir, S., Özgirgin, N., 2014. Wheelchair appropriateness in patients with spinal cord injury: a Turkish experience. Spinal Cord 52, 901–904. https://doi.org/10.1038/sc.2014.128
- Feller, R., Furin, M., Alloush, A., Reynolds, C., 2020. EMS Immobilization (Seated and Supine), in: StatPearls. StatPearls Publishing, Treasure Island (FL).
- Fogelberg, D., Atkins, M., Blanche, E.I., Carlson, M., Clark, F., 2009. Decisions and Dilemmas in Everyday Life: Daily Use of Wheelchairs by Individuals with Spinal Cord Injury and the Impact on Pressure Ulcer Risk. Top. Spinal Cord Inj. Rehabil. 15, 16–32. https://doi.org/10.1310/sci1502-16
- Hagen, E.M., 2015. Acute complications of spinal cord injuries. World J. Orthop. 6, 17–23. https://doi.org/10.5312/wjo.v6.i1.17
- Harrow-Mortelliti, M., Reddy, V., Jimsheleishvili, G., 2020. Physiology, Spinal Cord, in: StatPearls. StatPearls Publishing, Treasure Island (FL).
- Hassannejad, Z., Zadegan, S.A., Shakouri-Motlagh, A., Mokhatab, M., Rezvan, M., Sharif-Alhoseini, M., Shokraneh, F., Moshayedi, P., Rahimi-Movaghar, V., 2018. The fate of neurons after traumatic spinal cord injury in rats: A systematic review. Iran. J. Basic Med. Sci. 21, 546–557. https://doi.org/10.22038/IJBMS.2018.24239.6052
- Hausmann, O.N., 2003. Post-traumatic inflammation following spinal cord injury. Spinal Cord 41, 369–378. https://doi.org/10.1038/sj.sc.3101483
- Hoffman-Kim, D., Mitchel, J.A., Bellamkonda, R.V., 2010. Topography, Cell Response, and Nerve Regeneration. Annu. Rev. Biomed. Eng. 12, 203–231. https://doi.org/10.1146/annurev-bioeng-070909-105351
- Huebner, E.A., Strittmatter, S.M., 2009. Axon Regeneration in the Peripheral and Central Nervous Systems. Results Probl. Cell Differ. 48, 339–351. https://doi.org/10.1007/400_2009_19
- Jessen, K.R., Mirsky, R., 2016. The repair Schwann cell and its function in regenerating nerves. J. Physiol. 594, 3521–3531. https://doi.org/10.1113/JP270874
- Kaiser, J.T., Reddy, V., Lugo-Pico, J.G., 2020. Anatomy, Head and Neck, Cervical Vertebrae, StatPearls [Internet]. StatPearls Publishing.
- Moon, L.D.F., 2018. Chromatolysis: Do injured axons regenerate poorly when ribonucleases attack rough endoplasmic reticulum, ribosomes and RNA? Dev. Neurobiol. 78, 1011–1024. https://doi.org/10.1002/dneu.22625
- Nógrádi, A., Vrbová, G., 2013. Anatomy and Physiology of the Spinal Cord, Madame Curie Bioscience Database [Internet]. Landes Bioscience.
- Ottosen, C.I., Steinmetz, J., Larsen, M.H., Baekgaard, J.S., Rasmussen, L.S., 2019. Patient experience of spinal immobilisation after trauma. Scand. J. Trauma Resusc. Emerg. Med. 27, 70. https://doi.org/10.1186/s13049-019-0647-x
- Ouyang, H., Sun, W., Fu, Y., Li, J., Cheng, J.-X., Nauman, E., Shi, R., 2010. Compression Induces Acute Demyelination and Potassium Channel Exposure in Spinal Cord. J. Neurotrauma 27, 1109–1120. https://doi.org/10.1089/neu.2010.1271
- Patek, M., Stewart, M., 2020. Spinal cord injury. Anaesth. Intensive Care Med. 21, 411–416. https://doi.org/10.1016/j.mpaic.2020.05.006
- Popovich, P.G., Jones, T.B., 2003. Manipulating neuroinflammatory reactions in the injured spinal cord: back to basics. Trends Pharmacol. Sci. 24, 13–17. https://doi.org/10.1016/S0165-6147(02)00006-8
- Rahimi-Movaghar, V., Saadat, S., Vaccaro, A.R., Ghodsi, S.M., Samadian, M., Sheykhmozaffari, A., Safdari, S.M., Keshmirian, B., 2009. The efficacy of surgical decompression before 24 hours versus 24 to 72 hours in patients with spinal cord injury from T1 to L1 – with specific consideration on ethics: a randomized controlled trial. Trials 10, 77. https://doi.org/10.1186/1745-6215-10-77
- Rouanet, C., Reges, D., Rocha, E., Gagliardi, V., Silva, G.S., Rouanet, C., Reges, D., Rocha, E., Gagliardi, V., Silva, G.S., 2017. Traumatic spinal cord injury: current concepts and treatment update. Arq. Neuropsiquiatr. 75, 387–393. https://doi.org/10.1590/0004-282x20170048
- Silva, N.A., Sousa, N., Reis, R.L., Salgado, A.J., 2014. From basics to clinical: A comprehensive review on spinal cord injury. Prog. Neurobiol. 114, 25–57. https://doi.org/10.1016/j.pneurobio.2013.11.002
- Silver, J., Schwab, M.E., Popovich, P.G., 2015. Central Nervous System Regenerative Failure: Role of Oligodendrocytes, Astrocytes, and Microglia. Cold Spring Harb. Perspect. Biol. 7. https://doi.org/10.1101/cshperspect.a020602
- Theodore, N., Hlubek, R., Danielson, J., Neff, K., Vaickus, L., Ulich, T.R., Ropper, A.E., 2016. First Human Implantation of a Bioresorbable Polymer Scaffold for Acute Traumatic Spinal Cord Injury: A Clinical Pilot Study for Safety and Feasibility. Neurosurgery 79, E305-312. https://doi.org/10.1227/NEU.0000000000001283
- Tran, A.P., Warren, P.M., Silver, J., 2020. Regulation of autophagy by inhibitory CSPG interactions with receptor PTPσ and its impact on plasticity and regeneration after spinal cord injury. Exp. Neurol. 328, 113276. https://doi.org/10.1016/j.expneurol.2020.113276
- Wang, Y., Tan, H., Hui, X., 2018. Biomaterial Scaffolds in Regenerative Therapy of the Central Nervous System. BioMed Res. Int. 2018. https://doi.org/10.1155/2018/7848901
- Zhang, N., Yin, Y., Xu, S.-J., Wu, Y.-P., Chen, W.-S., 2012. Inflammation & apoptosis in spinal cord injury. Indian J. Med. Res. 135, 287–296.
References for Bioprinting
- Angela Faccendini et al. (2017) ‘Nanofiber Scaffolds as Drug Delivery Systems to Bridge Spinal Cord Injury’, Pharmaceuticals, 10(4), p. 63. doi: 10.3390/ph10030063.
- Choi, S.-W., Zhang, Y. and Xia, Y. (2010) ‘Three-Dimensional Scaffolds for Tissue Engineering: The Importance of Uniformity in Pore Size and Structure’, Langmuir, 26(24), pp. 19001–19006. doi: 10.1021/la104206h.
- Eshraghi, S. and Das, S. (2010) ‘Mechanical and microstructural properties of polycaprolactone scaffolds with one-dimensional, two-dimensional, and three-dimensional orthogonally oriented porous architectures produced by selective laser sintering’, Acta Biomaterialia, 6(7), pp. 2467–2476. doi: 10.1016/j.actbio.2010.02.002.
- Germain, L. et al. (2018) ‘3D-printed biodegradable gyroid scaffolds for tissue engineering applications’, Materials & Design, 151, pp. 113–122. doi: 10.1016/j.matdes.2018.04.037.
- Guarino, V., Causa, F. and Ambrosio, L. (2007) ‘Porosity and mechanical properties relationship in PCL porous scaffolds’, Journal of applied biomaterials & biomechanics: JABB, 5(3), pp. 149–157.
- Meco, E. and Lampe, K. J. (2018) ‘Microscale Architecture in Biomaterial Scaffolds for Spatial Control of Neural Cell Behavior’, Frontiers in Materials, 5, p. 2. doi: 10.3389/fmats.2018.00002.
- Thuaksuban, N. et al. (2011) ‘Biodegradable polycaprolactone-chitosan three-dimensional scaffolds fabricated by melt stretching and multilayer deposition for bone tissue engineering: assessment of the physical properties and cellular response’, Biomedical Materials, 6(1), p. 015009. doi: 10.1088/1748-6041/6/1/015009. Varntanian, S. (2017) ‘3D Printing of Polycaprolactone Scaffolds’. doi: 10.13140/RG.2.2.27949.28645.
- Wang, Y. et al. (2017) ‘Effective improvement of the neuroprotective activity after spinal cord injury by synergistic effect of glucocorticoid with biodegradable amphipathic nanomicelles’, Drug Delivery, 24(1), pp. 391–401. doi: 10.1080/10717544.2016.1256003.
- Wong, D. Y. et al. (2008) ‘Macro-Architectures in Spinal Cord Scaffold Implants Influence Regeneration’, Journal of Neurotrauma, 25(8), pp. 1027–1037. doi: 10.1089/neu.2007.0473.
- Wong, B. S., Teoh, S.-H. and Kang, L. (2012) ‘Polycaprolactone scaffold as targeted drug delivery system and cell attachment scaffold for postsurgical care of limb salvage’, Drug Delivery and Translational Research, 2(4), pp. 272–283. doi: 10.1007/s13346-012-0096-9.
- Zhang, Y. et al. (2018) ‘Melatonin for the treatment of spinal cord injury’, Neural Regeneration Research, 13(10), p. 1685. doi: 10.4103/1673-5374.238603.
References for Mussel Foot Protein
- Budisa, N. and Schneider, T., (2019). Expanding the DOPA universe with genetically encoded, mussel-inspired bioadhesives for material sciences and medicine. ChemBioChem. 20. pp2163-2190.
- Glass, P., Chung, H., Washburn, N., et al. (2010). Enhanced wet adhesion and shear of elastomeric micro-fiber arrays with mushroom tip geometry and a photopolymerised p(DMA-co-MEA) tip coating. Langmuir. 26(22). pp17357-17362.
- Haeshin, L., Lee, B.P. and Messersmith, P.B., (2007). A reversible wet/dry adhesive inspired by mussels and geckos. Nature. 488. pp338-341.
- Hwang, D. S.; Yoo, H. J.; Jun, J. H., et al., (2004). Expression of Functional Recombinant Mussel Adhesive Protein Mgfp-5 in Escherichia Coli. Appl. Environ. Microbiol.70.3352.
- Kan Y, Danner EW, Israelachvili JN, Chen Y, Waite JH, (2014) Boronate complex formation with Dopa containing adhesive protein retards pH-induced oxidation and enables adhesion to Mica. PLoS ONE 9(10): e108869.
- Lu, Q., Danner, E., Waite, J.H. et al. (2012). Adhesion of mussel foot proteins to different substrate surfaces. J R Soc Interface 10: 20120759.
- Menyo, M.S, Hawker, C.J. and Waite, J.H., (2013). Versatile tuning of supramolecular hydrogels through metal complexation of oxidation-resistant catechol-inspired ligands. Soft Matter. 9(43). doi:10.1039/C3SM51824H.
- Petrone, L., Kumar, A., Sutanto, C. et al. Mussel adhesion is dictated by time-regulated secretion and molecular conformation of mussel adhesive proteins. Nat Commun 6, 8737 (2015).
- Santonocito, R., Venturella, F., Piaz, F.D. et al., (2019). Recombinant protein Pvfp-5β: A potential tissue bioadhesive. J. Biol. Chem. 294(34). pp 12826-12835.
- Waite, J.H., (2017). Mussel Adhesion – Essential footwork. Journal of Experimental Biology. 220. Pp 517-530.
- Yu J, Wei W, Danner E, Ashley RK, Israelachvili JN, Waite JH. Mussel protein adhesion depends on interprotein thiol-mediated redox modulation. Nat Chem Biol. 2011;7(9):588-590. Published 2011 Jul 31. doi:10.1038/nchembio.630










