Human Practices
Our Values:
This year our main focus was to target the primary injury of spinal cord injuries. Presently, there are no available treatments on the market that can cure the primary injury of SCI. This lack of a proper solution motivated our team's project design in supplying a treatment that would both facilitate the patient’s recovery and provide them with a foundation towards functional rehabilitation. Our solution was motivated by the ethical limitations of treatments on the market targeting SCI, for instance, the risk of introducing growth factors and stem cells has not been fully investigated due to the insufficient amount of empirical data when studying the spinal cord.
The basis of our project design was expanded through the constant integration of our key stakeholders: medical experts, neurosurgeons, patients and industry personnel. We highly valued their advice as they supported our understanding of the moral, social and scientific implications of our design. In terms of our moral values, we believe it is vital to provide treatment for severe cervical spinal cord injuries, instead of subjecting them to a life of limited motor function. Renervate believes in sharing our findings with the scientific community in order to establish a more comprehensive understanding of the challenges faced in treating spinal cord injuries. Overall, we value the concept of community where every member of society should be involved with transparency in science communication.
The communities and resources that influenced our values:
Prior to approaching our stakeholders, we conducted extensive literature investigations; this theoretical infrastructure gave us direction to set up the backbone of our project. Particularly in terms of understanding the microenvironment of SCI lesions, the current use of scaffoldings and the protocols used in the protein purification of our mussel foot protein.
Our initial readings allowed us to pinpoint the medical challenges, which required further professional elucidation and ultimately determined our communication decisions. For example, our scaffold specification sheet for our scaffold design was guided by our literature research and the academics we interviewed. We verified our specifications were in line with the values of our product and this was achieved by ensuring every design decision was in line with the recommended specifications. Ultimately, resulting in the creation of an optimal version.
Holistic Design:
The Benefits of Holistic Therapies

In order to better understand holistic therapies, we sought out guidance from our main stakeholders, patients presenting with spinal cord injuries. In order to inform ourselves with the ethical implications of holistic therapies, we attended a Cafe event organised by the Spinal Injuries Association. It is a platform for people with SCI to speak about their daily life. We were humbled for the opportunity to attend a session and gain valuable input directly from people with SCI. Among many things, we learned that a range of holistic therapies is helpful in treating some comorbidities, which develop from living with SCI long term. For example, massage therapies relieve tightness in the shoulders and also provide means to socialise with others. Whilst, supplements, such as glucosamine, provide benefits for joint health and help particularly with wear and tear in the shoulders. Similarly, shockwave treatments are also helpful in fibrosis in the shoulders and calcification of tendons. Finally, swimming was found to be really impactful as it helped strengthen the body and relieve tension.
We recognise that if some of these therapies if administered incorrectly, can do more harm than good. Therefore, it is essential that the individuals consult their GP prior to starting any holistic treatments. Ultimately, however, holistic therapies and our approach are not mutually exclusive; we aim for our approach to be used alongside these holistic therapies where they are needed. Nevertheless, we hope that by restoring the function of the spinal cord, the need for these therapies reduces as wear and tear of the shoulders, which develops when living long term with SCI, for example, is less likely. You can learn more about how they advised us towards our clinical guidance below!
Using Proteases
We considered the potential therapeutic efficacy of proteases in treating SCI and promoting neurite outgrowth. This novel approach provides a less invasive way to treat the glial scar, potentially relieving the need for its resection. Proteases can greatly decrease the release of growth inhibitory molecules in the lesioned area thereby creating a more permissive environment for neuronal growth. However, upon more research we identified several limitations, which are summarised in SCI Pathophysiology. Further investigations are needed to assess their effectiveness in treating smaller lesions.
Reducing Surgeries

Primary injury to the tracts in is irreversible whilst the secondary injury, which is caused by the release of cytokines, is reversible (Alizadeh et al., 2019). Therefore, current treatments aim to prevent and mitigate secondary damage to the spinal cord, which necessitates an assessment of anatomical stability and physiological management. Surgical interventions are normally carried out in patients with acute SCI who suffer from progressive neurological deficit due to the compression of the spinal cord or have a dislocation-type injury to the spinal column. Currently, there are no guidelines regarding the optimal timing of surgical interventions (Rath and Balain, 2017). Where the patient requires such surgery to decompress or stabilise the spinal cord, we aim for our scaffold to be implanted in the same surgery so a second one is not required.
Implementing a Biodegradable Design
Our approach involves the insertion of our scaffold within the spinal cord, which after the resection of the glial scar, will bridge the cavity. By providing means for axon guidance and promoting axonal growth through topology and topography, the scaffold will create a more permissive environment for neuronal growth. Through this regenerative phase of axons, our scaffold will retain its mechanical integrity, as its degradation rate follows the timescale for axonal regeneration. The use of a biodegradable scaffold eliminates the need for its surgical resection.
Shaping our project through Human Practices:
Throughout our research, we constantly checked back with our stakeholders to ensure we were on the right path. Initially, we approached medical experts specialised in the microenvironment of SCI lesions as we had to ensure we were creating a focused scaffold design. This also guaranteed our scaffold design would not further exacerbate the spinal cord injury, ensuring we are only providing an improved treatment that will ameliorate a patient’s quality of life. Secondly, we collaborated with academics to validate our mussel foot protein modelling, which informed us on the technical implications of our project and above all confirmed we were prioritizing the right protocols for the purification of our protein. Thirdly, we thoroughly evaluated and brainstormed the development of our scaffold design with academics who provided technical advice on which macro and microarchitectures would compromise our project’s aim and further validated that our scaffold is pertaining to the challenge of building a bridge to facilitate axonal regrowth. Finally, we approached patients with SCI to discuss their experience in living with an injury and how this has impacted their quality of life. This opportunity informed us of the importance of meeting the requirements of the patient by improving motor ability whilst sustaining an ethical approach.
The creation of Renervate is owed to the clinical, academic, industrial and above all personal guidance we received in order to develop a comprehensive and understanding solution. This is the story of how we have incorporated a variety of different perspectives into our project, in order to ensure we have supplied a project that is good for the world.
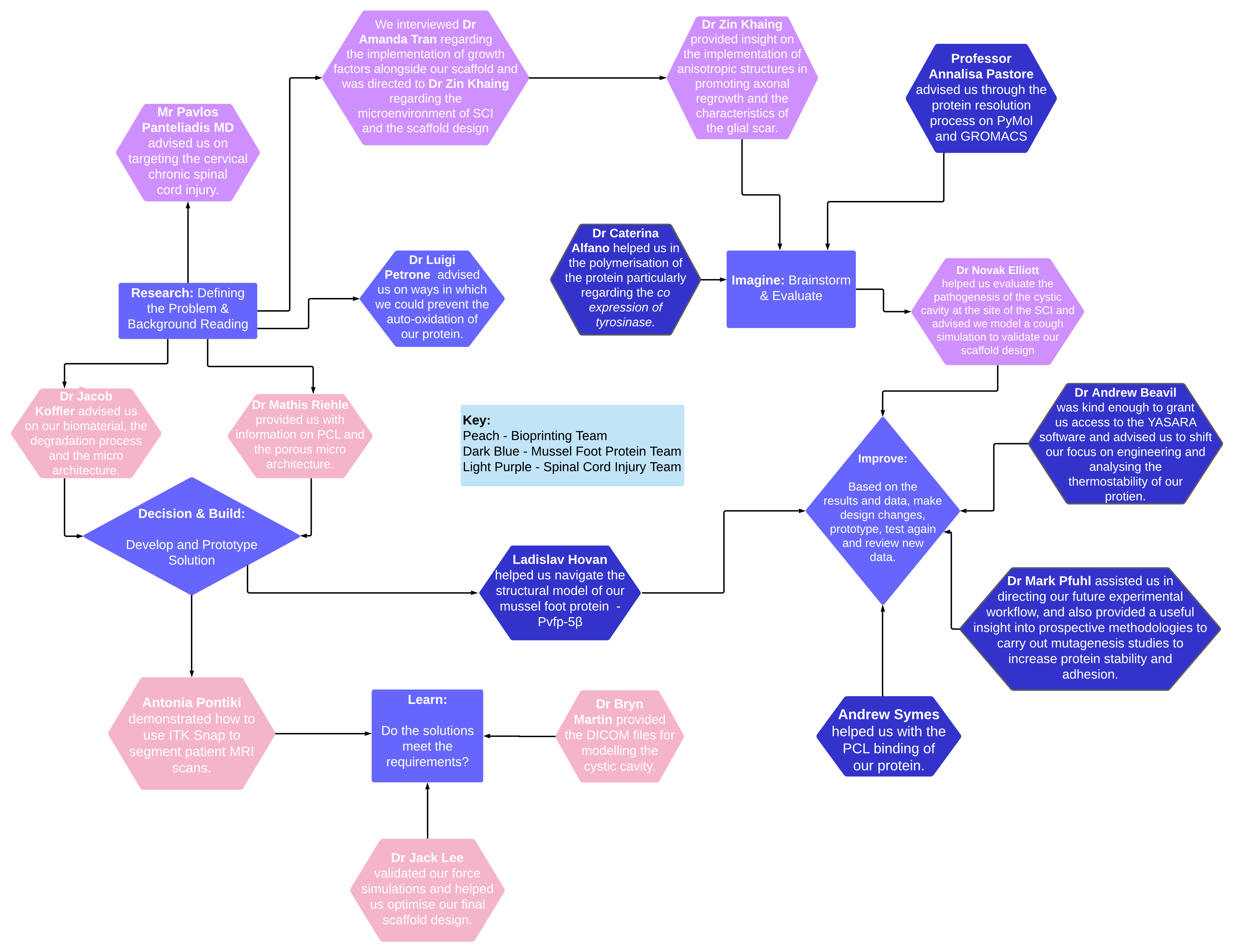
Integrated Human Practices
Collaborating with our stakeholders allowed for our understanding of the ethical, technical and communication decisions required for the development of a comprehensive project design. The numerous ethical limitations of treating spinal cord injuries had to be evaluated and the most pertinent ones were highlighted before proceeding with our research. These limitations included the limited scope of present treatments, their invasive and expensive nature and lastly the risk of worsening a patient’s circumstances.
We utilised the technical advice of our stakeholders to target these ethical implications and applied the best safety measures respecting their higher knowledge. We eliminated most of the unethical aspects involved in our project to prioritise the wellbeing of our patient subset. For example, we did not use growth factors alongside our scaffolding, due to limited access to the lab to gather further validation on their function alongside the risk of axons only growing on the scaffolding, instead of spreading to our target tissue, as advised by a medical expert.
We decided what values we needed to apply to our research by focussing on our intended outcome of providing a novel, inexpensive and non-invasive treatment for spinal cord injuries. We ‘closed the loop’ between our design and expected results by determining if we had stayed true to our original plan, incorporated our stakeholders' contribution and focussed on the creation of a project that is approachable and less daunting for our patient subset.
Acting Upon Academic Guidance:
The Spinal Cord Injury Subgroup:
The Spinal Cord Injury Team approached medical experts in order to clarify the characteristics of the microenvironment present at the site of injury. This was an essential step in our research to ensure we were using our scaffold to ameliorate the current approach of treatments on the market. These conversations helped us inform our communicative and technical decisions regarding our project design, guiding us towards building a project that resolves the current absence of a complete SCI treatment.

Dr Amanda Tran is a Brain and Spinal Cord Regeneration Scientist at Seattle Children's Hospital, her paper on the ‘Regulation of autophagy by inhibitory CSPG interactions with receptor PTPσ and its impact on plasticity and regeneration after spinal cord injury’ provided the foundation for our research into the implementation of growth factors in our scaffold design. Her background in spinal cord regeneration allowed for a discussion on the logistics of our project, highlighting the importance of understanding the risk of opening up the dura and removing the chronic glial scar at the lesion epicenter in order to fit our scaffold inside the cavity. She confirmed that extracellular matrix molecules like HSPGs, which could be used to outcompete CSPG interactions, are chemoattractants in vitro. After this discussion, we evaluated the surgical implementation of our scaffold and decided against using any growth factors because of their risk in making our scaffold a perfect target for cells to grow instead of a highway to direct them to our target tissue. Dr Amanda Tran directed us towards an excellent bioengineer and SCI specialist, Dr Zin Khaing who is an Assistant Professor of Neurological Surgery at the University of Washington.
We approached Dr Zin Khaing towards the beginning of our research into the effects of topography and topology on axonal regrowth; she helped consolidate our knowledge on the microenvironment of the glial scar and provided a foundation for further research into anisotropic surfaces. She described the importance of alignment to achieve mechanical support and reinforced the notion of using appropriate internal architectures to avoid the occurrence of axonal bundles. Dr Zin Khaing advised we have a focussed but simple design for our scaffold to avoid any confusion in the communication of our solution. She highlighted the dynamic nature of spinal cord injuries and how our proposed implementation should target this - we adapted our design to her advice by concluding that we would need to resect the glial scar prior to placing our scaffold as the scar creates a barrier where there would be no interaction between our scaffold and the surrounding environment. Through the resection of the lesion, we would be creating a more manageable glial scar. Furthermore, she clarified the importance of using a scaffold material that did not degrade into lactic acid due to its adverse effects. Henceforth, we validated the use of PCL which degrades into caproic acid (Bliley and Marra, 2015).
The 3D Bioprinting Subgroup:
The 3D Bioprinting Team focussed on conducting interviews that would help propel our initial design into a final model. We evaluated our approach and consulted academics in order to validate our decisions regarding micro and macro architecture selection, modelling degradation and simulating our designs in computer-aided design softwares.
Dr Mathis Riehle, who is Director of the Centre for Cell Engineering at the University of Glasgow, provided us with information on PCL as a biomaterial. Prior to selecting our scaffold material, we approached him so we could brainstorm and evaluate the limitations and advantages of PCL in comparison to other materials. In addition, he helped us communicate through the combined PCL-chitosan approach, the immune response and the impact of using ECM proteins alongside different materials. Riehle stated that the consistency of the spine is similar to cream cheese. Therefore, we researched and adapted our mechanical properties of our scaffold design according to his advice. Ultimately, we chose PCL because it has a Young’s Modulus similar to that of the spinal cord, it is able to be 3D bioprinted and to refine our decision he suggested we avoid using a chitosan-collagen blend as this would elicit an immune response. Additionally, Riehle stated that the scaffold could have microstructures as a part of its implementation, eg. pores and grooves. This provided a foundation for our research into anisotropic surfaces in promoting axonal regrowth and was achieved through the use of Rhinoceros with the Grasshopper extension.

We approached Dr Jacob Koffler, an Assistant Professor of Neurosciences at the University of California San Diego, for assistance on our scaffold design, it’s micro-architecture and degradation modelling. He advised us on the best way to create pores, which is to ensure that the printing of the scaffold is compatible with the method of creating pores. This meant we had to evaluate our proposed pore sizes and microarchitecture, as our bioprinter would have to have a resolution within 100-600 microns to successfully print microstructures. Dr Koffler also guided us on the use of PCL as a bioink. He said that we should ensure that our PCL is clean of solvent when printing and verify that our specifications match what is needed when implanting it within the spinal cord.
Lastly, Dr Koffler stated that the scaffold should last at least 6 months, in order to achieve proper axon bridging. Thanks to his guidance we conducted our degradation modelling, which demonstrated that our scaffold would last for approximately 36 months. His advice allowed us to shape a more focal scaffold design, that allowed for the investigation of axonal binding through topology and topography.

Once we had completed our initial modelling of our scaffold design and characterised the microenvironment of the cavity present in spinal cord injuries, we approached Dr Novak Elliott, who is an Adjunct lecturer in the Department of Mechanical Engineering at Curtin University. After reading his thesis, ‘Mathematical Modelling and Analysis of Cerebrospinal Mechanics: An Investigation Into the Pathogenesis of Syringomyelia’, we understood we required further clarification on how the cranial-spinal pressure dissociation theory was the most supported theory for the formation of the cavity formed in cervical spinal cord injuries. He then confirmed we should complete further modelling of our scaffolding through a cough/sneeze simulation to demonstrate the mechanical forces working on the spinal cord. Additionally, he advised us on the parameters and constraints we needed to impose on our scaffold - predominantly ensuring the rostral and caudal part of the scaffold do not move. He helped inform us on how to make a more realistic simulation that would properly demonstrate the values of our solution.
Dr Novak Elliott was also kind enough to direct us towards Dr Bryn Martin , who is Vice President of Research, Precision Delivery, and Cerebrospinal Fluid Sciences at Alcyone Lifesciences, Inc. and was able to provide us with the relevant DICOM files to model the syrinx in the cervical spinal cord for the validation of our procedure. As a result of this, we used data from Dr Bryn Martin's literature to apply a pressure load radially on our open path with core scaffold in Autodesk Inventor and this ultimately allowed us to create a more realistic model of our project design.
In order to validate our scaffold design we needed further insight into the implementation of segmentation softwares. We spoke to Antonia Pontiki who is a PhD student at King’s College London. Her expertise in the field of Biomedical Engineering helped us with using the segmentation software, ITK Snap. After our meeting with her we were able to obtain MRI scans of patients with spinal cord injury and successfully segmented the lesion at the trauma site. This allowed us to adjust the dimensions of our open path with core scaffold, improving it’s technical implementation as it can now be personalised to each patient’s individual case. Subsequently, this enabled us to ‘close the loop’ between our design and what is required by each patient to achieve optimal recovery.
We approached Dr Jack Lee, who is a lecturer at King's College London, to evaluate our proposed scaffold design and verify our force simulations. He informed us that we had used the appropriate method for our simulations and authenticated our researched scaffold designs based on the mechanical properties alone. Dr Lee advised us that the central portion of our open path with core scaffold was too thin and would degrade more rapidly compared to the end sections. We therefore chose to reconfigure our scaffold, creating a thicker design to ensure it would last longer. This advancement of our treatment will optimise our scaffold design in ensuring it does not degrade prior to our patient’s recovery.



The Mussel Foot Protein Subgroup
Without a great deal of academic guidance, the mussel foot protein (MFP) portion of our project would not have been possible. Since the beginnings of our research, we have had to seek help to allow us to determine how we could use Molecular Dynamics (MD) simulations to explore the structure of our protein as well as how we could use this knowledge to further our understanding of polymer creation. Furthermore, we have employed guidance from a variety of academics regarding how we could create a safe, non-immunogenic polymer and have integrated this knowledge into our protocols. Finally, we have been very grateful to those who have guided us with our mutagenesis studies such as using iGAM and the YASARA software. Hereunder, we will outline those who have been fundamental in shaping our project and the ways in which they did so.

Professor Annalisa Pastore works as a researcher in structural and molecular biology, specialising in proteins found in neurodegenerative disease. She is a group leader at UK DRI at King’s and a Professor of Chemistry & Molecular Biology at King's College London. After first reading the publication “Recombinant mussel protein Pvfp-5β: A potential tissue bioadhesive”, we decided to get in touch with Professor Pastore as she was not only one of the authors of the paper but is based at King’s. Through her, we were put in touch with Dr Caterina Alfano . Both researchers became so fundamental to our project and understanding of our protein that they came to be Supervisors for our project. At the start of our project, Professor Pastore provided us with insight into the methods of purifying our protein, which we have incorporated into our protocol and will use next year during the second phase of our project. As a consequence of the pandemic, she was key in the transformation of our project from one that was primarily wet-lab to one that was entirely in silico.
As we decided to create a bioadhesive polymer, she described how we could carry out structural modelling to better understand the relationship between the structure and function of our protein. Therefore, our team began to use MD simulations and GROMACS to gain greater insight into how our protein adheres to surfaces and how this could be employed in our system. She further helped us understand the structure of our protein, emphasising the importance of the disulphide bridges in the strength and adhesion of the protein. This shaped our structural modelling and ensured that we concentrated on ensuring our disulphide bridges were in the appropriate positions and of the necessary number. To do so, she directed us to experts such as Erika Földesné Dudás, who throughout our entire protein resolution process on PyMol and GROMACS helped us with disulphide bridge resolution. Finally, she shaped our experimental design and workflow that will be employed next year through her advice regarding chemically treating the protein both for polymerisation, storage and adhesion.

Through Professor Pastore, we were put into contact with Dr Caterina Alfano, Group Leader in Structural Biology and Biophysics at Fondazione Ri.MED: Palermo. Dr Alfano was integral to our understanding of the protein and the design of our BioBricks. Having successfully expressed and purified Pvfp-5β, she taught us how to create an optimal expression system. We began with the issue of polymerisation. We were having trouble finding a way of retaining the adhesive properties of the protein by protecting the dopamine groups, as well as polymerising the protein, which involved modification to the dopamine residues. Dr Alfano directed us to the paper she and her lab had published on how to accomplish both adhesion and polymerisation.Her lab had used a co-expression system with tyrosinase which polymerises the protein using dopamine residues through a dopaquinone intermediate. Once the cysteinaldopa link has been made, dopamine is restored, therefore ensuring maximal adhesion. This information was critical and we decided to co-express our protein BioBrick expression vector with a tyrosinase enzyme to create our polymer. She was fundamental in how we designed our expression system. Additionally, Dr Alfano assisted us with the process of chemically treating our protein, thus guiding our future experimental workflow.
She was also able to direct us to Dr Erika Földesné Dudás who helped supervise our model simulations on GROMACS. Dr Dudás, a postdoctoral researcher at King’s College London, played an important role during the initial stages of our modelling process of Pvfp-5β. We came in contact with Erika through Professor Annalisa Pastore, who recommended Dr Dudás as a chaperone for running model simulations using GROMACS. Most importantly she supported us with her advanced bioinformatics knowledge. Assistance with preparation our protein’s PDB files by editing our sequence ID's, to be ready for our model simulations - while also recommending that we run a minimisation simulation to complete our protein model in the first place!
Professor Annalisa Pastore also put us in contact with Dr Ladislav Hovan, a theoretical chemist with expertise in Biophysics and Molecular Dynamics, to provide assistance in resolving the structural model of our mussel foot protein, Pvfp-5β. Through aiding us in navigating the more complicated areas of the GROMACS MD package, Ladislav was able to help us elucidate a particular disulphide bridge on our protein model that we had struggled with. This was achieved by first attempting to forcibly impose the bridge by altering the distance at which the GROMACS software recognises a disulphide bond. However, this was not ideal as it unlinked the other disulphide bridges across the protein model. Through successive simulations after imposing, and un-imposing our desired disulphide bond, the residues involved were brought to a close enough distance in which we could use the YASARA graphical modelling software to link the residues through a 'spring to atom' forcefield constant. By helping us generate our sought after disulphide bond, Dr Hovan allowed us to further understand our protein and his advice allowed us to ameliorate the technical proposed implementation of our project design.
After identifying the adhesion mechanism that takes place between the mussel foot protein and the substrate surface, we quickly realised that the adhesive bonds form in acidic conditions created by the hydrogen ions the mussel excretes. Under alkaline, seawater pHs the mussel foot protein is unable to form the required adhesive bonds due to the auto-oxidation of DOPA residues. We understood that this would be problematic to our project as the DOPA residues would also auto-oxidise under physiological pHs. After reading a paper by Dr Lugi Petrone, who is a senior scientist specialising in antifouling coatings for boats, that mentioned how Pvfp-5β had intrinsic antioxidant properties. We decided to further investigate this by asking for his professional advice, to which he replied suggesting chelating the -OH groups of DOPA with ferric ions, thus potentially preventing oxidation of the DOPA residues. He also mentioned how we could potentially free DOPA from the ferric ions by changing pHs - this was pivotal as it gave us the idea of having an on and off switch for the catechol protecting group.
As our structural model progressed, we began to seek out ways in which we could best use our structural model. This research lead us to watch the After iGEM seminar “Dry Lab Impulse #1: Team Calgary 2019 - iGAM Software for evaluating protein modifications”.
Check out the seminar hosted by Andrew below!

Our initial aim was to use our structural model to determine which residues could be changed to increase the adhesiveness of our protein. Therefore, we were very intrigued to come across this seminar, which was given by Andrew Symes, a Bachelor’s student at the University of Calgary and the Co-Founder and CFO at yOIL Technologies. After watching the After iGEM seminar, we decided to get in touch with Andrew to see if the software could be used in our project. Initially, we believed that it would be ideal to use iGAM to predict which mutations would result in increased adhesion in our protein. We discussed this with Andrew, and decided to research into a fitness function that could be applied to the iGAM genetic algorithm. He guided us in our research and explained to us not only which residues would be most ideal to modify but which software we could use to investigate them further. However, we came to recognise that there was limited literature regarding the adhesion mechanism of our chosen protein, Pvfp-5β (at the time of researching, a recent publication has provided novel insights into this isoform and we will utilise this knowledge in the second phase of our project). With no access to the lab, we recognised that we would be unable to construct a fitness function for the adhesive behaviour of Pvfp-5β. Therefore, we carried out physicochemical analysis into Pvfp-5β to determine which aspects of the protein we could alter through iGAM using various web servers. The results of this investigation revealed to us that our protein has a very low predicted protein half-life, hence we decided to look into this further.
Yet, after speaking with Dr Andrew Beavil (see below) we realised that this would be incomplete without experimental data and decided to continue to examine our final structural model with the Yasara software. Although we did not have enough time to develop a fitness function this year that we could use with iGAM, we will do so next year and will carry out the wet lab validation of our chosen mutant. We are so grateful for Andrew’s help with preparing the algorithm for our particular protein.

Dr Andrew Beavil was kind enough to grant us access to the YASARA software - which enabled us to begin physicochemical analysis on our protein. He helped guide us in the next steps of our protein analysis - by informing us that the extension of our protein half life was not possible without wet lab data, we were then able to shift our focus onto engineering and analysing the thermostability of our protein.To further our understanding of how we could examine and modify the adhesiveness and thermostability of Pvfp-5β, we reached out to Dr Mark Pfuhl, who is Reader in Cardiovascular Structural Biology at King’s College London. After discussing our structural model with Dr Pfuhl, he informed us of using consensus modelling to carry out semi-rational protein engineering. As a consequence of the expanse of preexisting knowledge regarding MFPs from several mussel species, we decided to incorporate this in silico method of protein engineering into our in silico and computational work with Pvfp-5β. Although we were unable to complete this during the Phase I of our project, we will implement this into our modelling alongside iGAM next year. Additionally, he provided us with insight into how we could use experimental, wet lab approaches to carry out semi-rational protein design. In particular, Dr Pfuhl described phage display assays which can be designed to determine which mutations maximise protein interactions. As a consequence of the applications of these assays to our protein isoform, we have decided that we will look into this experimental approach during our wet lab next year.
The Inclusivity Subgroup:
The Inclusivity Team approached experts specialised in ethics in order to shape their work towards creating a safer, more inclusive platform for students entering the STEM and synthetic biology field as well as guide us in conducting ethical research throughout our project .
At the beginning of our explorations into how we can make iGEM and STEM more inclusive, we decided to carry out a pilot study in who is and who isn’t being represented in our community. The aims of this pilot study were to gain an overview of which identities are not being represented so that we can design an action plan to help increase their presence. We firmly believe that the more diverse STEM is, the better it will be. Therefore, we wanted to identify if there are any gaps in the iGEM community. Through our pilot study, we were put into contact with Dr Anne S. Meyer, an Associate Professor at the University of Rochester Department of Biology, as well as the iGEM Diversity and Inclusion Committee. Their feedback was fundamental in ensuring that we appropriately designed our pilot study. Our discussion with Dr Meyer allowed us to modify our initial methodologies to make it more inclusive and more open-ended to ensure all identities could be represented. Through her advice, we decided to get in touch with Dr Anne McKee, who would further shape our survey-based research. Dr Meyer also helped guide the design of our Biologix Competition. She supported our ideas and showed us areas where we could improve, such as starting more locally to have a smaller impact initially.
We approached Dr Anne McKee, who is the Postgraduate Research (PGR) Lead in the Centre for Education and a senior lecturer of medical education at King’s College London, for advice on attending the spinal cord injury cafe. Our conversation allowed us to evaluate the moral implications of sharing our research with patients and the importance of using the correct language when addressing them. She also assisted us with our pilot study, where she informed us about safe practices of conducting a survey, collecting people’s data and the universities protocol on obtaining feedback. She helped inform us on the steps we needed to take in creating a project that is comprehensive and good for world by advising us to seek the help of the ethics board prior to releasing an official survey.
Acting Upon Clinical Guidance:

During the course of our initial research on the pathophysiology of SCI, we had an opportunity to briefly speak to Mr Pavlos Panteliadis MD, a consultant spinal surgeon at Guy’s and St. Thomas' Hospitals in London, UK. We have received guidance on the anatomy of the spinal cord and surgical access to the cervical part of the spine, which allowed us to conclude that, in theory, it would be possible to develop a scaffold for cervical SCI. Additionally, we were informed about the microenvironment and the pH of the cyst that may develop at the site of injury, although this conversation made it clear that specialist research groups need to be consulted instead for more advanced understanding of the biochemistry involved in the SCI - we were directed to Professor Elizabeth Bradbury's work on SCI and aim to be in contact with her in phase two.
Additionally, we visited the KCL medical school to conduct several dissections on the spinal cord. This experience allowed us to better investigate the anatomy of the spinal cord, giving us a more realistic perspective on spinal cord injuries. Similarly, it further developed our moral and scientific values by allowing us to visualise the impact of our project on a patient’s life. We approached the project with a new found confidence, particularly involving the pathways of the spinal cord, thanks to the guidance provided by the teaching staff of the Dissection Lab at KCL.
Engagement with the Spinal Injuries Association:

The Spinal Injuries Association is a non-profit organisation set up by individuals with spinal cord injuries with the focus on developing support networks and representing the wider SCI community through education and outreach. Our team was given the privilege to sit in on one of the Zoom Cafe afternoons where we have gained insight on the following topics:
- Daily management of spinal cord injury & adjustments when first diagnosed
- Advice for new members of support systems and carers
- The importance of holistic therapy approach in treating SCI
- The effect of COVID-19 on individuals with SCI
- The importance of language when describing individuals living with SCI
- Accessibility and the portrayal of SCI patients in media
Prior to attending the meeting, we established that it was our moral and scientific duty to put the experience and needs of individuals living with SCI on the forefront of our project. It is not sufficient to focus only on the pathophysiology of the injury - the SCI cafe meeting gave us an insight on the wider societal context of the implications of the SCI. It allowed us to establish a dialogue with patients in order to fully understand their experience of living with SCI and the importance of using our platform to spread awareness. They consulted us on the importance of sustaining a proper routine and planning ahead to ensure that their injury is not getting the best of them, while sharing their encouragement towards innovation and looking into new technologies that can be used to help them live with the condition - the primary caveat to this is the cost of new solution, which reinforced the necessity of ensuring that our scaffolding is marketed as an inexpensive solution. This resonates with the core social and scientific values of our solution - its design makes it financially accessible, while being uniquely tailored to the requirements of the patient’s injury.
Participants of the meeting also educated us on the importance of language and terminology around SCI, particularly how varied of a description individuals living with SCI choose for themselves. We learned about the social model of disability and how it has created an environment where society is the factor that disables an individual and not their impairment. For example, describing someone as wheelchair bound implies that they are dependent on the wheelchair whereas a wheelchair user is given more control. We were informed of the necessity of encouraging education of word choice - specifically how different places, languages and individuals will refer to disabilities differently. As a team, we utilised this information to firstly educate ourselves and then our audience by publishing infographics and additional education materials on our social media platforms that highlight the importance of our word choice.
You can watch our discussion with the Spinal Injuries Association below!
Acting upon Industry Guidance:
We consulted several experts in the fields of medtech and legislation in order to ensure that in closing the loop between what we have designed and what was desired, we were founding a product that is responsible and good for the world. The following individuals guided us through the procedures we would have to follow as well as the importance of biological safety in releasing our product into the market.


In order to complete our understanding regarding the legislative process to acquire the CE marking we contacted Dr Rachel Sparks, a Lecturer at King’s College London. Along with the legislative guidance to obtain the CE marking for medical devices, she advised us on ways to research safety issues our scaffold could have and explained a few assessments we should consider carrying out: biological safety, mechanical forces, sterility and long term biocompatibility. This was essential in evaluating whether our product was responsible and conforms to current laws.
Furthermore, to further our knowledge regarding the process behind getting the CE marking we had a meeting with Professor Prashant Jha, Head of Affordable Medical Technologies at King’s College London. He broke down the process for the CE marking into several points and suggested how we might tackle each of the points to finally apply for the CE marking. He also expanded on the clinical investigation section, which would be the next step we would have to tackle in certifying our product. We also discussed the principal milestones of our clinical investigation to reach human trials.
Due to the FDA's global influence, we decided to research the basic steps we would have to follow to become FDA approved. For this purpose we approached Dr Jim Sterling, Professor at Keck Graduate Institute to discuss our project. He introduced the concept of predicate devices to us which could be implemented as a possible shortcut to receiving FDA approval. The shortcut involved using a similar product to ours previously approved by the FDA to avoid clinical trials. However, in a meeting with Dr Travis Schlappi, Assistant professor at Keck Graduate Institute, we concluded that in our case the predicative device procedure could not be utilised, since due to the use of our novel MFP which would be designed by us, we would have to utilise clinical trials to prove the safety of our MFP. Furthermore, we were able to discuss the implementation of our synthetic polymer with Dr. Jaime Lazúen Alcón, who suggested a protocol we could follow to prove that our protein is non immunogenic in the clinical investigation.
Complementary to our scientific research, we completed research into the barriers in STEM. We approached Tobias Wingbermuehle, Co-Founder Clustermarket & Founder of Science Entrepreneur Club for advice regarding the creation of our competition, Biologix. Our competition was created in response to the lack of opportunities for high school students in getting free opportunities in STEM and synthetic biology. He advised us on our communication decision by directing us towards his marketing team in order to advertise our project and spread awareness around the subject matter. Thus, we has shaped our Science Communication and Education methodologies. In the future, we will be discussing how we could collaborate with the Science Entrepreneur Club further to share our message. Through our newly founded KCLSU society, we will be working closely with his organisation over the next year to host events for King's College London's students.
Scientific Integrated Human Practices Overview:

References:
- Alizadeh, A., Dyck, S.M., Karimi-Abdolrezaee, S., 2019. Traumatic Spinal Cord Injury: An Overview of Pathophysiology, Models and Acute Injury Mechanisms. Front. Neurol. 10, 282. https://doi.org/10.3389/fneur.2019.00282
- Bliley, J.M., Marra, K.G., 2015. Chapter 11 - Polymeric Biomaterials as Tissue Scaffolds, in: Vishwakarma, A., Sharpe, P., Shi, S., Ramalingam, M. (Eds.), Stem Cell Biology and Tissue Engineering in Dental Sciences. Academic Press, Boston, pp. 149–161. https://doi.org/10.1016/B978-0-12-397157-9.00013-8
- Rath, N., Balain, B., 2017. Spinal cord injury—The role of surgical treatment for neurological improvement. Journal of Clinical Orthopaedics and Trauma 8, 99–102. https://doi.org/10.1016/j.jcot.2017.06.016













