OVERVIEW

To address biocontainment concerns, we set ourselves to devise a system to prevent the survival of our yeast outside of their intended growth vessel. Though our bioreactor was carefully designed to tightly contain the biological components, we wanted to create a biocontainment system as a cautionary measure to ensure the safety of the greater environment and community. In order to develop our biocontainment strategy, we followed the engineering cycle, as described below:
- Understand the problem
- Research and Ideate Solutions
- Design Solutions: experimental design
- Create and test: proof of concept
- Apply: Shift to Y. lipolytica
Though the cycle appears to be linear, in actuality it will be a circular process, with findings from different stages of the cycle informing the workflow of prior steps. We spent this season conducting extensive literature review, speaking to experts in academia, and actually working in the lab to test a proof of concept for our system. Following this iGEM season, we will again apply this engineering cycle as we apply our proof of concept to engineer and test Y. lipolytica for our biocontainment system.
UNDERSTANDING THE PROBLEM
Why is biocontainment important?
Synthetic biology is an emerging field that promises scalable and low-cost solutions to global health, agricultural, and environmental problems in the form of self-replicating biological devices. As these solutions increasingly become a reality, they migrate from tightly regulated laboratories to private spaces, such as households. This raises significant safety concerns regarding biocontainment, as the introduction of non-native organisms into the environment can result in unintended adverse effects on the ecosystem. Oviita is a community product that is meant to be cultivated outside of the laboratory by the people in the community, as such ensuring high levels of biosafety was a crucial part of our project. In order to ensure safety, iGEM Calgary set out to design a robust, yet sustainable biocontainment system.
Previous strategies
When we began our journey towards finding an effective biocontainment strategy, we considered many different options including auxotrophy and a kill switch system, before eventually settling on our final strategy of a complementary auxotrophy co-culture system.
Auxotrophy prevents the production of a vital molecule by an organism, thereby forcing the organism to become reliant on the external supplementation of the molecule of interest for survival (Torres et al., 2016). A positive side to auxotrophy is the low mutation rate of reverting the auxotrophy back to prototrophy. However, such a system introduces high costs, and is ultimately unsustainable because of the need for supplementation.
An alternative biocontainment strategy is the introduction of a kill switch system. Such a system comes with its own positive aspects and limitations. Kill switch systems are genetic circuits that lead to the production of a toxin or inhibitory molecule following exposure to a certain stimulus, resulting in the death of the organism (1). For example, a kill switch system could involve the production of a nuclease in response to blue light. However, some limitations for this method is that the system relies upon the availability of the stimulus in order to be effective. In addition, when we talked to Dr. Robert Mayall, he explained how there is a higher rate of mutating out the kill switch system due to the fitness cost instilled in an organism.
RESEARCH AND IDEATE SOLUTIONS
Our Goals
While there is no such thing as an ideal biocontainment system, our goal is to ensure that our system is as secure as possible. For our purposes, a system based of auxotrophy is the most feasible. Therefore, our system must be:
- Easy to maintain and self-sustaining
- Auxotrophy is expensive to maintain, so we want to minimize costs. It also cannot be too sensitive, as we do want yeast growth within the system. Therefore, common cues, such as environmental cues, would not be apt at regulating the system
- Have low possibility of horizontal gene transfer
- Horizontal gene transfer would result in the auxotrophic strains picking up genes that would allow them to synthesize the molecules that they currently cannot, resulting in the co-culture containment strategy becoming useless
- Ensure that organisms cannot still survive after escape to the environment
- This system was developed to ensure the organisms stay within the system; if environmental amino acid levels are sufficient to maintain life, the biocontainment system would not be as effective as desired
Initial ideas
The first biocontainment strategy we considered was a light-induced kill switch system. We reasoned that since light is readily available in the environment, and we don’t want our yeast cells to survive outside of the growth vessel in the environment, light would be a viable option to induce the death of our yeast cells. When we were exploring this option, we first came across an inducible kill switch system where exposure to blue light results in the expression of Cas9. Specifically, we wanted Cas9 to target essential genes of the yeast, as cleavage of these genes would result in the death of the organism. We found a system by Kuwana et al. (2015), which involves a photoreceptor system of two magnets (pMag and nMag) that each are linked to one-half of the Cas9 gene. In response to blue light, the two magnets join together, allowing Cas9 to join together and cleave the target gene.
However, upon speaking to Dr. Marija Drikic, a metabolomics research associate, we decided to pivot from this for our biocontainment strategy. Her concerns included the fact that in her experience with research, the whole process in order to result in the cleavage of the gene of interest had numerous areas that could go wrong. For example, the cas9 protein might take a lengthy time to be expressed--too long of a time to be suitable for a biocontainment system.
Based on this feedback, we went back to literature review to find an alternative strategy. Eventually, we came across a different light inducible system by Xu et al. (2018), which instead involves the expression of a transcription factor that is sensitive to light. In response to light, the transcription factor binds to its binding region, and causes the expression of the GOI. We decided to explore whether the GOI could be toxic protein that could thus be expressed in response to light. However, in speaking to Dr. Zaramberg, a professor specializing in yeast research, we learned that there were a number of difficulties with the successful implementation of this idea. For example, whatever toxin we chose had to be non-toxin to humans or denatured by heat. As well, leaky expression of the protein has to be controlled for, as any premature expression would result in the killing of our yeast cells. Though we did extensive literature review, we unfortunately could not find a toxin that had previously been heterologously expressed with sufficient characterization or testing. We received advice, once again by Dr. Marija Drikic, who suggested we research further into proteins or enzymes that could target components of the yeast cell wall, protein inhibitors and nucleases. After considering several options we decided upon nucleases as our GOI to be expressed by our cells in response to light. We reasoned that this would be the simplest means for the yeast to be killed.
In conjunction with our light inducible promoter system, we started looking into yeast toxins that would kill off the cell. Ultimately, we came across the idea of using nucleases, as mentioned by Balan and Schenberg (2005). They had developed a system where under certain environmental conditions, nuclease activity would be activated and cause death of the cell. While nucleases are typically secreted into extracellular media, this system ensured these nucleases were secreted inside the cell such that apoptosis would be induced. The nuclease that we chose would have to fit two criteria. First, it would have to have a small GC-rich recognition site. Second, it would have to be toxic to the yeast and non-toxic to humans, such that it would serve as an effective biocontainment system but not harm people who ingested it. We started looking into many different nucleases, searching for one that would function at an optimal temperature and target essential sites in the yeast cell.
However, upon introduction of our finalized biocontainment strategy to faculty members, we received a number of comments about our system that we decided to completely switch gears. For instance, if our cells were ever released into the environment, we were questioned on whether light would be able to penetrate soil or deep water bodies in order to reach the escaped organisms. As well, light can be a very variable environmental condition. Thus, there were concerns on whether light could be relied upon as our inducible agent on a consistent basis to activate our kill switch system. Based on these concerns, we decided to go back to the drawing board.
One of the other biocontainment strategies we started exploring was the toxin-antitoxin system. These systems usually comprise two expression systems; a toxin expression system and an antitoxin expression system. The toxin would have a lethal effect on the cell upon expression but the antitoxin would be able to diffuse the toxin effect on the cell. The antitoxin would be under the control of a weak constitutive promoter while the toxin would be under the control of an environmentally inducible promoter. Low expression of the antitoxin would buffer out any leaky expression of the toxin but once a certain environmental cue activates the toxin expression system, the toxin would be produced in significant quantities compared to the antitoxin and would be able to exert its lethal effect in the cell.
This biocontainment strategy has a major advantage over the typical kill switch system. Kill switch systems are most often evolutionary unstable. The leaky expression of the toxin in these systems even when the system is turned off would result in reduced fitness of the cells containing the kill switch system. As such, there is an evolutionary pressure for these switches to be selected out of the population. The toxin-antitoxin system, however, would reduce the fitness cost associated with the toxin expression system as an antitoxin would be expressed to buffer out leaky expressions of the toxin. Therefore, the toxin-antitoxin systems tend to be more evolutionary stable.
Despite some of the advantages of this biocontainment method, there are still serious shortcomings associated with this strategy. Our discussion with Dr. Robert Mayall, helped us identify some of the challenges of the toxin-antitoxin system. One of the major downsides of using such a system is the extra stress we would put the cells under by engineering two unnecessary pathways into the cells. In particular, the antitoxin expression pathway needs to always be active which would significantly reduce the fitness and growth rate of the host cell. Although some improvements have been made in recent years in this field, these systems are not yet close to the robust and viable biocontainment strategy we are looking for. Second, such systems have shown to have mild levels of success only in bacteria since they are native to those organisms. The introduction of these exogenous systems in yeast would be challenging and have not shown to be effective to the best of our knowledge. Third, reliance on a specific environmental signal to activate the toxin system is not always viable, especially since our yeast is designed to be used in all kinds of communities with varying environmental conditions. As such, we decided to look for a more viable biocontainment strategy.
Finalized strategy
Benefits of Auxotrophy
Dr. Robert Mayall, one of our HP contacts, helped us determine the benefits of auxotrophy. For one, auxotrophy is much more stable than a killswitch. Killswitches tend to produce some type of harmful molecule to the cell, even with measures to prevent leaky expression. On the other hand, auxotrophy does not produce a harmful molecule to the cell, decreasing the possibility of it mutating out. An issue with auxotrophy is that they require constant supplementation. Implementing a co-culture system circumvents this issue and provides a sustainable approach to biocontainment.
Syntrophic Co-culture of Auxotrophs
We wanted to create a biocontainment system that took advantage of the robustness of an auxotrophy system but one that did not have the sustainability issues most associated with an auxotrophic system. Our discussion with Dr. Robert Mayall led us to the idea of using a cross-feeding co-culture of auxotrophic organisms. In this system, each strain will be auxotrophic for the molecule the other strain can produce, resulting in cross-feeding between the two strains. This eliminates the need to continuously add amino acid supplements to the culture and at the same time, it ensures tight regulation of the organisms as they would be mandated to stay together in the bioreactor to obtain their required amino acid. In case microorganisms escape the bioreactor, they would not be able to survive as they require their partner in sufficient quantity to grow, a condition only met inside the bioreactor. Thus this allows us to design a robust yet sustainable biocontainment strategy.
In order to bring our new biocontainment strategy to fruition, we looked in the literature for the mechanism of microorganism cross-feeding in co-cultures. We found many successful previous cross-feeding experiments done using different bacteria species such as S. indica, B. subtilis, and E. coli. In fact, such cooperative interaction in bacterial communities is thought to be prevalent in nature as such bacteria already have some of the mechanisms required for setting up successful cooperative relationships with other members of the community.
Yeast, however, are not naturally very good at exchanging metabolites with one another. A study by Shou et al. found that metabolite exchange among co-growing yeast cells are not occurring at growth-relevant quantities. Thus, two complementary auxotrophs would not be able to supports each other in a co-culture. However, they also found that a small set of genetic modifications in the biosynthesis of the amino acids could help establish cooperation between the two auxotrophic strain. These modifications would allow the yeast strains to overproduce the amino acid required by the other co-culture and thus would allow them to establish a cooperative community. In particular, they engineered an adenine auxotrophic strain of S. cerevisiae to overproduce lysine for a lysine auxotroph strain of S. cerevisiae that would overproduce adenine in return. The engineered complementary strains were able to form stable communities where a minimum cell density of each strain was required to support the growth of the co-culture. Another study by Hoek et al. performed similar experiments but with a tryptophan auxotrophic, leucine overproducing strain, and a leucine auxotroph, tryptophan overproducing strain of S. cerevisiae. They found that not only the two strains were able to establish a viable cooperative community but also over time the two strains established an obligate mutualism relationship where each strain required its partner for proliferation. The mentioned studies highlight the ability of yeast to take part in synthetic cross-feeding communities where they become completely dependent on their partner strain for growth.
S. cerevisiae strains
Based on the previously conducted research regarding the complementary cross-feeding yeast strains, we were interested in acquiring a pair of the complementary yeast strains to characterise and test the effectiveness of a co-culturing system for ourselves. Luckily for us, the Murray lab at Harvard University graciously sent us the two W303 S. cerevisiae strains they had engineered,:
- ymm60: the leucine auxotroph which overproduces tryptophan
- ymm65: the tryptophan auxotroph that overproduces leucine.

Figure 1: Auxotrophic Co-culture diagram of ymm60, leucine overproducing and tryptophan auxotrophic, and ymm65, tryptophan overproducing and leucine auxotrophic strains
Specifically, each strain was engineered to overproduce the amino acid by introducing a gene for that amino acid that was resistant to feedback inhibition (Muller et al., 2014). The ymm60 strain was engineered with a feedback resistant form of the LEU4 gene, while the ymm65 strain was engineered with the feedback-resistant version of the TRP2 gene. Additionally, to aid in monitoring the growth of the yeast strains during co-culturing experiments, each strain includes the expression of a fluorescent protein. The leucine overproducing strain expresses the mCitrine protein, while the tryptophan overproducing strain expresses the mCherry protein. Both strains also express the cyan fluorescent protein, yCerulean.
EXPERIMENTAL DESIGN
Proof of concept
Our decision to work primarily on a proof of concept using S. cerevisiae was based on the advice of Dr. Peter Facchini, a biochemistry professor at the University of Calgary who believed it would be more practical for us to establish that our system worked in an already well-characterized chassis. As the Murray Lab agreed to provide us with a pair of tryptophan overproducing/leucine auxotrophic and leucine overproducing/tryptophan auxotrophic strains, we were set! These strains had been successfully co-cultured in the past, but never used for their applications to biocontainment. We decided to confirm auxotrophy, characterize amino acid overproduction, establish a co-culture, and finally, run environmental experiments to ensure that the environmental amino acid concentration would not be sufficient to allow for yeast colony propagation in the event of escape. If we were successful in these steps and this system was established to have a future as a viable biocontainment system, we would be able to shift this system to work in Y. lipolytica.

Figure 2: Biocontainment Workflow
Auxotrophy
We designed some simple experiments to confirm that the W303 engineered strains, ymm60 and ymm65, were indeed auxotrophic for tryptophan and leucine respectively. Both engineered strains along with the control wildtype W303 strain were each plated on three types of media:
- SD media containing all amino acids except for leucine
- SD media containing all amino acids except for tryptophan
- SD media containing all amino acids
Growth of the strains at 30 degrees Celsius on different plates were be observed for 48 hours.
Amino acid overproduction
We wanted to confirm the overproduction of the amino acids by the two engineered strains. To do this, we based our experimental protocol to that of a bioassay used by Shou et al. (2007) and Zhang et al. (2014), where they measured the concentration of amino acid released into the media by yeast and bacteria, respectively.
Essentially, this involved us first creating a standard curve of OD600 measurements of the auxotrophic W303 strain grown in different concentrations of amino acid for 24 hrs, for leucine and tryptophan, respectively. Based on the OD600 value present at the end of the 24 hrs, we could then use this curve to approximate the concentration of amino acid released into the media.
Once the standard curves were constructed, we set ourselves to test the concentration of leucine or tryptophan released to the media by the following strains:
- Engineered strains:
- ymm60 : Leu+, Trp-
- ymm65: Trp+, Leu-
- Control strains:
- LEU2 transformed W303 strain
- TRP1 transformed W303 strain
The original W303 strain is already auxotrophic for both leucine and tryptophan. Thus, to make our respective control strains, we transformed the W303 strain with the PRS415 centromeric plasmid containing the Leu2 gene, and the PRS414 centromeric plasmid containing the Trp1 gene, respectively so that we could create our two control strains and thus compare the concentration of amino acid produced by a strain engineered to overproduce the amino acid to a strain that is only producing baseline or normal levels of that amino acid.
To determine the concentration of amino acids released into the media, we first grew cultures of various time lengths of the four different strains in media deficient for the amino acid it is producing. The cultures include those grown to mid-log, 24 hrs, and 48 hrs. Once the cultures were grown, they were filtered to remove the presence of cells, so only the media was present.
Following this, a 96-well plate was filled with samples of the media from the four yeast cultures inoculated with the W303 strain. We measured the OD600 values of the growth of the W303 cells in these samples in a plate reader over a 24 hr period. Based on the OD600 value measured at the end of the 24 hr period, we used the standard curve previously constructed to determine the approximate amount of amino acid (leucine or tryptophan) released into the media by the four strains. Our goal was to compare the engineered strain to its corresponding control strain to verify that the concentration of amino acid produced by the engineered strain was in fact greater than the control strain.
The experiment was completed in triplicate. OD600 measurements were recorded in 15-minute intervals over a 24 hour period using a 96-well plate reader at 30° C. A more detailed protocol for this can be found here.
We wanted to characterize the performance of the ymm60 and ymm65 strains in the co-culture. Therefore, we design a co-culturing experiment based on a study done by Shouk et al. These experiments served as a proof of concept that our engineered strains are indeed able to support each other’s growth by cross-feeding the amino acids required by the other strain and that they are capable of working cooperatively together to form stable syntrophic communities.
To start the co-culture, an equal amount of mid-log phase ymm60 and ymm65 strains were added together in 2ml of SD media. We suspected that the strains would initially need a bit of help before they could establish a mutualistic relationship with their fellow partner in the co-culture. That is why we grew the co-cultures in 4 different SD mead with varying starting levels of leucine and tryptophan. This would allow us to determine the initial requirements for a successful co-culture. The following four SD media were the treatments used for the co-cultures:
- Zero: SD media containing no leucine or tryptophan
- Low: SD media containing 1.0 uM tryptophan and 8.0 uM leucine
- Medium: SD media containing 4.0 uM tryptophan and 32 uM leucine
- High: SD media containing 8.0 uM tryptophan and 64 uM leucine
Note: ratio of tryptophan to leucine in the SD media was 1:8 since leucine is required 8 times more than tryptophan by the cells. This would ensure equal footing for both strains in the co-culture.
Once the co-cultures were started, they were allowed to grow for 2 days, and their growth was monitored every 24 hours by measuring three different variables; the OD600, fluorescence at 610nm, and fluorescence at 587nm. The OD600 would give an approximation of the total cell density of the co-culture while the fluorescence measurements would allow us to monitor the growth of each strain individually. This is because each strain is engineered to produce a different fluorescent protein. The fluorescence measurement for each protein could be used to infer the relative success of the strains in the co-culture. Below is the list of strains used in the experiment and their associated fluorescent protein.
- ymm60 (Trp-, Leu++) → mCitrine (emision wavelength: 610nm)
- ymm65 (Trp++, Leu-) → mCherry (emission wavelength: 587nm)
The entire point of a biocontainment system was to prevent the organism from causing damage to the environment in the event of escape. Therefore, we had to ensure that environmental concentrations of amino acids would be insufficient for the yeast to thrive on. This way, the yeast co-culture would be killed off in the environment and remain reliant on the conditions inside of the bioreactor. When in nature, there are two primary elements: earth and water. We needed to ensure that our yeast co-culture wouldn’t survive in either environment, so we collected some soil and river environmental samples and got to work.
The environmental samples we used were river water, autoclaved river water, garden soil, and autoclaved garden soil. The autoclaved samples primarily tested for nutrients already present in the environment without accounting for the fluxes due to living microorganisms.
The non autoclaved samples tested for the effect of living microorganisms and other, more sensitive, nutrients present in the environment that may be degraded due to high temperatures. As our strains were auxotrophic for leucine and tryptophan respectively, we wanted to check specifically for the presence of these amino acids in the environment.
For ymm60 (leucine overproducing and tryptophan auxotrophic), the treatments were:
- SD-Trp- + autoclaved bow river water
- SD-Trp- + non autoclaved bow river water
- SD-Trp- + autoclaved soil water
- SD-Trp- + non autoclaved soil water
- YPD (positive control)
- YPD + autoclaved bow river water
- YPD + nonautoclaved bow river water
- YPD + autoclaved soil water
- YPD + nonautoclaved soil water
For ymm65 (tryptophan overproducing and leucine auxotrophic strain), the treatments were:
- SD-Leu- + autoclaved bow river water
- SD-Leu- + non autoclaved bow river water
- SD-Leu- + autoclaved soil water
- SD-Leu- + non autoclaved soil water
- YPD (positive control)
- YPD + autoclaved bow river water
- YPD + nonautoclaved bow river water
- YPD + autoclaved soil water
- YPD + nonautoclaved soil water
The treatments including YPD served as positive controls, while the treatments involving SD media served to supply the nutrients other than the amino acids each strain was auxotrophic for. This way, we could determine whether the environmental samples had sufficient amounts of amino acids to sustain yeast strain growth. Each of these treatments had a blank, where no yeast was suspended. The treatments with yeast were also done in triplicate and grown over the course of 60 hours in a 96-well plate. For a more in-depth look at exactly what we did, check out the protocols.
RESULTS
Auxotrophy

Figure 1. Auxotrophy test, showing lack of growth of the ymm60, ymm65, and wildtype W303 strain on non-permissive (amino acid deficient condition) (left) and growth on permissive conditions (supplemented with amino acids) (right).
We successfully characterized the auxotrophic natures of our engineered strains. The ymm60 strain (tryptophan auxotroph) grew on all the plates except for the tryptophan deficient plate and the ymm65 strain (leucine auxotroph) grew on all plates except for the tryptophan deficient plate as expected. This confirms the auxotrophy of our engineered strains as the strains were not able to grow on the media lacking the amino acid they are auxotrophic for but they showed growth on media supplemented with the required amino acids. The wildtype W303 strain did not grow on either the leucine or tryptophan deficient media. This is expected as wildtype W303 strain is auxotrophic for both leucine and tryptophan.
Amino acid overproduction
Standard curve preparation
Following OD600 measurements at the end of a 24 hr period after inoculation of media with W303 S. cerevisiae cells for media containing varying concentrations of leucine and tryptophan respectively, we were able to construct the following standard curves:

Fig 1. Standard curve of OD600 vs leucine concentration following growth of W303 S. cerevisiae cells in SD-leu media containing varying concentrations of leucine after 24 hrs (0, 3, 6, 9, 12, 15 mg/L).

Fig 2. Standard curve of OD600 vs tryptophan concentration following growth of W303 S. cerevisiae cells in SD-trp media containing varying concentrations of tryptophan after 24 hrs (0, 0.5, 1, 1.5 mg/L).
Leucine amino acid overproduction
After collecting OD600 measurements over a 24 hour period for the growth of the W303 strain in the media from the 24 hr, and 48 hr cultures of the ymm60 strain and Leu2-transformed strain, the following results were obtained:

Fig 3. OD600 measurements for the growth of a W303 S. cerevisiae strain in filtrate from 24 hr cultures of Leu-transformed control strain, and ymm60 engineered strain. Measurements were taken at 15-minute intervals over a 24-hr period at 30° C.

Fig 4. OD600 measurements for the growth of a W303 S. cerevisiae strain in filtrate from 48 hr cultures of Leu-transformed control strain, and ymm60 engineered strain. Measurements were taken at 15-minute intervals over a 24-hr period at 30° C.
When analyzing the OD600 data collected, it appears that the engineered ymm60 strain did in fact release greater concentrations of amino acid into the media compared to the control strain (figure 1, 2). This verifies that the engineered strain is successfully overproducing leucine. To estimate the concentration of leucine released to the media by each strain, the OD600 measurement taken at the end of the 24 hrs period was interpolated to the standard curve previously constructed that showed OD600 vs leucine concentrations (figure 1):
Table 1. The OD600 values and corresponding concentration of leucine released to the media by each of the strains after 24 hrs. Measurements were taken for the filtrate from 24 hr, and 48 hr cultures of the Leu-transformed and ymm60 strains.
|
|
||
|
Strain |
OD600 |
Leucine produced (mg/L) |
|
Leu-transformed |
0.0665 |
0.111 |
|
ymm60 |
0.0739 |
0.508 |
|
48 hour cultures |
||
|
Strain |
OD600 |
Leucine produced (mg/L) |
|
Leu-transformed |
0.0552 |
-0.487 |
|
ymm60 |
0.0624 |
-0.109 |
When comparing the leucine concentration released to the filtrate by the engineered and control strain from the 24 hr cultures, it can be seen that the engineered strain produced almost five times more leucine to the media compared to the control strain (table 1). However, when analyzing the data from the 48 hr cultures, negative values were obtained (table 1). We believe that the yeast cells may have been dying due to a lack of sugar in the media, and thus unable to release excess leucine to the media. To test this theory, we hope to repeat the same experiment but with a 48 hr culture that is supplemented with extra glucose during the culturing period. We would also like to conduct the experiment using 96-hr cultures that are supplemented with glucose to observe the production of leucine by the strains over a longer period of time.
Tryptophan amino acid overproduction
After collecting OD600 measurements over a 24 hour period for the growth of the W303 strain in the media from the 24 hr, and 48 hr cultures of the ymm65 strain and Trp1-transformed strain, the following results were obtained:

Fig 5. OD600 measurements for the growth of a W303 S. cerevisiae strain in filtrate from 24 hr cultures of Trp-transformed control strain, and ymm65 engineered strain. Measurements were taken at 15-minute intervals over a 24-hr period at 30° C.

Fig 6. OD600 measurements for the growth of a W303 S. cerevisiae strain in filtrate from 48 hr cultures of Trp-transformed control strain, and ymm65 engineered strain. Measurements were taken at 15-minute intervals over a 24-hr period at 30° C.
When looking at the data for tryptophan overproduction, we received unexpected results. Though ymm65 is engineered to overproduce tryptophan, according to our data it appears that the control strain actually produces more tryptophan to the media. This pattern was observed for both the 24 hr and 48 hr cultures. To determine the reasoning behind this, we first considered that perhaps the starting OD600 value was greater in the control strain, thus making the results appear as though the control strain had a greater final OD600 compared to the experimental strain, even though that difference could be accounted for by the difference in initial values. However, this reasoning does not appear to hold. When analyzing the initial OD600 values measured for the 24 hr cultures, the filtrate for both cultures exhibited similar starting OD600 values (table 2).
Table 2. Initial OD600 values measured for the filtrate from the 24 hr cultures.
|
Strain |
Starting OD600 value |
|
Trp-transformed |
0.0415 |
|
ymm65 |
0.042 |
Thus, we recognized that the difference in OD600 values observed between the control and engineered strain was indicating a difference in tryptophan produced by the two strains. Conversing with Laura Sosa, a graduate student in a yeast research lab, we were able to think of two possible reasons for our results.
When we analyzed the genotype of the W303 S. cerevisiae strain (the original strain we had transformed with Trp1 to make our control strain), we were able to make a key observation.
Genotype W303: MATaura3-1 his3-11,15 leu2-3,112trp1-1 ade2-1 can1-100
This genotype tells us the genetic background of the strain. The genotype tells us that the W303 strain is auxotrophic for Leu2 and Trp1. This is why to make our control strain, we transformed with Trp1, so that it would produce baseline levels of tryptophan. However, in speaking to Laura Sosa, she pointed out that according to the genotype, the mutation to result in the tryptophan auxotrophy was a point mutation. A point mutation has a high probability if inadvertently reverting back to its original form of the gene. Thus, we propose that potentially the Trp1 mutation reverted back, resulting in tryptophan production by the strain. However, since we also transformed the strain with a plasmid containing the Trp1 gene, this would likely result in the overproduction of tryptophan to the media. To test this theory, we can streak the untransformed W303 strain on media deficiency in tryptophan and see if we observe any growth. If growth is observed, that means the mutation reverted back.
A second possibility for the overproduction of tryptophan by the Trp-transformed control strain, is due to a difference in promoter strength. The Trp1 gene was introduced to the W303 strain in the form of the centromeric (single-copy) plasmid, PRS414. This plasmid includes a constitutive promoter for the expression of the Trp1 gene. Conversely, the feedback-resistant Trp2 gene may possess a promoter of lower strength, thus accounting for the difference in amino acid production by the two strains. To test this theory, we are currently in the process of contacting the researchers who engineered the strains to learn more on the promoter integrated into the engineered ymm65 strain. Comparing the promoter strengths from the engineered strain to the promoter on the plasmid that we introduced to the W303 strain, will indicate whether this reasoning holds.
The table shown below shows the estimated concentration of tryptophan released to the media by the control and engineered strains from the 24 hr and 48 hr cultures (table 3). The results convey the same pattern observed from the growth curves outlined above, with greater amounts of tryptophan released by the control strain compared to the engineered strain (table 3).
Table 3. The OD600 values and corresponding concentration of tryptophan released to the media by each of the strains after 24 hrs. Measurements were taken for the filtrate from 24 hr, and 48 hr cultures of the Trp-transformed and ymm65 strains.
|
24 hour cultures |
||
|
Strain |
OD600 |
Tryptophan produced (mg/L) |
|
Trp-transformed |
0.100 |
0.296 |
|
ymm65 |
0.0808 |
0.148 |
|
48 hour cultures |
||
|
Strain |
OD600 |
Tryptophan produced (mg/L) |
|
Trp-transformed |
0.100 |
0.296 |
|
ymm65 |
0.070 |
0.0616 |
Conclusions
Overall, we were able to successfully confirm the overproduction of leucine by the ymm60 engineered strain. Though we were unable to obtain similar results showcasing the overproduction of tryptophan by the ymm65 engineered strain, we were able to exercise our critical thinking skills and reason out the basis for the discrepancy, which was still a valuable experience. Going forwards, we will conduct the appropriate steps to investigate the source for the tryptophan production results.

Figure 1. OD600 curve for 2ml co-culture of ymm60 and ymm65 strains over a two-day growth period. Co-cultures were treated with SD media containing varying amounts of leucine and tryptophan. Lecien and tryptophan concentrations increased from 0 to a maximum of 8.0 uM tryptophan and 64 uM leucine from Zero to High treatment. Two replicates were used for each treatment.
The OD600 measurement of all the treatments increased over the two day growth period. Since OD600 is an indirect measurement of cell density, these results suggest that the co-culture as a whole grew over time from a starting point of around 0.7 to OD600 values of 0.8 to 0.85. A key observation is that our co-cultures are showing growth even at 0 concentrations of leucine and tryptophan (Zero treatment) which might indicate that despite what we originally thought, an initial supplement of amino acid might not be required to get the co-culture going. Interestingly, our data might also suggest that the treatments Zero and Low are showing higher growth rates compared to the Medium and High treatments. This is counterintuitive as the richer media are expected to facilitate higher growth rates.
Figure 2. Mean mCherry fluorescence (left) and Mean mCitrine fluorescence (right) for 2ml co-culture of ymm60 and ymm65 strains over a two-day growth period. mCherry was excited at 587nm and measured at 610nm, while mCitrine was excited at 514nm and measured at 529nm. Co-cultures were treated with SD media containing varying amounts of leucine and tryptophan. Lecien and tryptophan concentrations increased from 0 to a maximum of 8.0 uM tryptophan and 64 uM leucine from Zero to High treatment. Two replicates were used for each treatment.
OD600 would show us the growth of the co-culture as a whole but it is not possible to tell whether the observed growth of the co-culture is due to the proliferation of one of the strains or both strains by looking at the OD600 measurements alone. That is why we also monitored the fluorescence produced by each strain separately. Both strains produce a different fluorescence protein; ymm60 expresses mCitrine while ymm65 expresses mCherry. The fluorescence values of each protein is an indirect measurement of the abundance of the strain that produces that fluorescence protein. This is because as the number of cells grows, there would be more cells to produce the fluorescent protein, therefore, the total fluorescence value of the culture should also increase. Based on the fluorescence curve of ymm60 and ymm65 we can see that both cultures have shown growth over the two-day growth period for all the treatments, indicated by an increase in fluorescence of both mCitrine and mCherry protein. However, it is difficult to compare fluorescence signals from the curves as mCitrine has inherently a higher brightness compared to mCherry. Hence a much stronger signal observed for mCitrine. In order to normalize the data and allow for a better comparison of the relative growth of the two strains, we compared the percent change in fluorescence over the two-day growth period.

Figure 3. Percent change in mCherry fluorescence (left) and mCitrine fluorescence (right) for 2ml co-culture of ymm60 and ymm65 strains over a two-day growth period. mCherry was excited at 587nm and measured at 610nm, while mCitrine was excited at 514nm and measured at 529nm. Co-cultures were treated with SD media containing varying amounts of leucine and tryptophan. Lecien and tryptophan concentrations increased from 0 to a maximum of 8.0 uM tryptophan and 64 uM leucine from Zero to High treatment. Two replicates were used for each treatment.
We can see that for Zero and Low treatments both strains are proliferating. Each strain has shown an increase in fluorescence of at least 15% over the two-day growth period. The same however cannot be said about the Medium and High treatments. For the High treatment, both strains have a relatively small change in their fluorescence (less than 5%), indicating that both of the strains had close to no growth. The Medium treatment shows the ymm65 strain dominating as it has a significantly higher percent change in the fluorescence compared to almost zero change observed for the ymm60. This goes against our original idea a richer starting media would result in higher growth of the strains.
In order to help understand our data better, we turned into the literature. Hoek et al. performed a similar experiment using complementary auxotrophs of S. cerevisiae . Interestingly, he also observed a similar trend in data to ours. The co-cultures appeared to be cooperative and have developed an obligate mutualism relationship at zero to low levels of provided amino acid supplements. However, once the amino acid content of the media increased to mid/high levels, the strains showed competitive behaviour, ultimately resulting in one strain dominating. This helps explain the observed trend in our data. At Zero and Low treatments. The initial amount of amino acid provided is small, resulting in strains needed to develop a competitive relationship with one another in order to survive. This is because the survival of each strain is dependent on the other strain in the co-culture. Therefore, it is in the best interest of both strains to cooperate together as shown by the considerable growth of both strains in the co-culture. As the concentration of provided amino acid increases in Med and High treatments, strains would no longer be dependent on one another and could use the free amino acid provided in the media. Therefore, the strain would not cooperate and form a competitive relationship for the nutrients in the media which was evident in the Med treatment where ymm65 was dominant in the co-culture at the expense of ymm60
Conclusion: The most important takeaway from our data is that despite what we originally thought, the strains do not need an initial boost in form of supplemented amino acid to produce a successful co-culture. Without any given leucine or tryptophan, the two strains started cooperating right away and formed a stable syntrophic community as evident by both the fluorescence and OD600 data. Our time in the lab was unfortunately limited due to the restrictions set upon us by the Coronavirus pandemic, however, in the future, we hope to perform even more experiments on the co-cultures as our current data shows are very promising.

Fig 1. Growth of ymm60 strain in various environmental conditions, including YPD positive control, mixture of YPD with autoclaved river water, nonautoclaved river water, autoclaved soil water, nonautoclaved soil water, and a mixture of SD-Trp- media with autoclaved river water, nonautoclaved river water, autoclaved soil water, and nonautoclaved soil water. Each bar represents the average of 3 replicates. Error bars are +/- standard deviation.

Fig 2. Growth of ymm65 strain in various environmental conditions, including YPD positive control, mixture of YPD with autoclaved river water, nonautoclaved river water, autoclaved soil water, nonautoclaved soil water, and a mixture of SD-Leu- media with autoclaved river water, nonautoclaved river water, autoclaved soil water, and nonautoclaved soil water. Each bar represents the average of 3 replicates. Error bars are +/- standard deviation.
The OD600 measured indicates the overall yeast growth overtime. The YPD positive controls, as expected grew the most, demonstrating the growth of the yeast in optimal conditions. The half YPD - half environmental sample media also had substantial yeast growth, although not nearly as much as the solely YPD media. This was indicative of the environmental samples having less nutrients than found in the YPD media.
The ymm60 strain only grew in the autoclaved river water sample without external tryptophan supplementation, while the ymm65 strain grew in both autoclaved river water and non autoclaved river water without external leucine supplementation. Neither of the soil samples exhibited positive growth for non-supplemented ymm60 or ymm65, while the positive control for ymm60 also did not display growth.
The strains that are stated to have no growth in the graphs actually produced negative results, with the experimental strains having a lower OD than the blanks. The OD for the experimental strains did not decrease, but the blank readings did go up over time in very small quantities. We hypothesize that the presence of native microorganisms in the environmental samples may have increased the readings for the blanks to a greater quantity than the yeast was able to grow, as those microorganisms were present in their native environment. This ultimately resulted in data that was not applicable to the purposes of this experiment.
According to the error bars, the growth of ymm60 was significantly less in the autoclaved river water than the positive control demonstrated. The error bars for the ymm65 treatments indicate the same, with unsupplemented samples growing significantly less than supplemented samples. The error bars for the ymm65 treatments are also much smaller than those for the ymm60 treatments, indicating a higher level of accuracy in the ymm65 experiments. Regardless, the yeast cells in unsupplemented media show much lower growth, if not no growth whatsoever, compared to YPD-supplemented yeast cells in environmental samples.
Conclusions
In conclusion, environmental conditions significantly hinder the growth of the ymm60 and ymm65 strains individually, if not prevent growth altogether. This could be due to a variety of factors, such as insufficient available amino acids, or other microorganisms that outcompete these strains. Due to a lack of laboratory availability, we weren’t able to collect all the data necessary to determine whether environmental conditions would prevent growth or just hinder growth, but these results demonstrate a promising start! In the future, we would also have to run environmental tests on the co-culture of ymm60 and ymm65, to determine whether they could survive together in the wild.
SHIFTING TO Y. LIPOLYTICA
Design considerations
The Auxotrophs
For the choice of auxotrophy in Yarrowia lipolytica we talked with Dr. Vanina Zaremberg who is a yeast expert. She mentioned that the point of a good auxotrophic system is to be effective at prohibiting cell growth when a certain metabolite is not supplemented and be stable so it does not get mutated out of the cell. Based on her comment and information found in the literature, we decided on leucine and tryptophan as our auxotrophic molecules. These two amino acids are required by the cells in high quantity and therefore, serve as strong regulatory controls on the growth of the organism. They also have a low revert back rate, as deletions made in this pathway have shown to be stable. We decided to target the deletion of two commonly deleted genes in the pathways of leucine and tryptophan auxotrophs. For leucine, a deletion of LEU2 gene, 3-isopropylmalate dehydrogenase, has shown to produce stable auxotrophs in both Y. lipolytica and many other yeasts such as S. Cerevisiae. Tryptophan auxotrophy has not been introduced in Y. lipolytica yet to the best of our knowledge. In order to make tryptophan auxotrophic strain of Y. lipolytica , we plan to target TRP1, a common gene targeted in S. cerevisiae tryptophan auxotrophs. Since the tryptophan biosynthesis pathway of S. cerevisiae and Y. lipolytica are almost identical, knockout of TRP1 should produce tryptophan auxotrophs in Y. lipolytica.
Amino Acid Overproduction
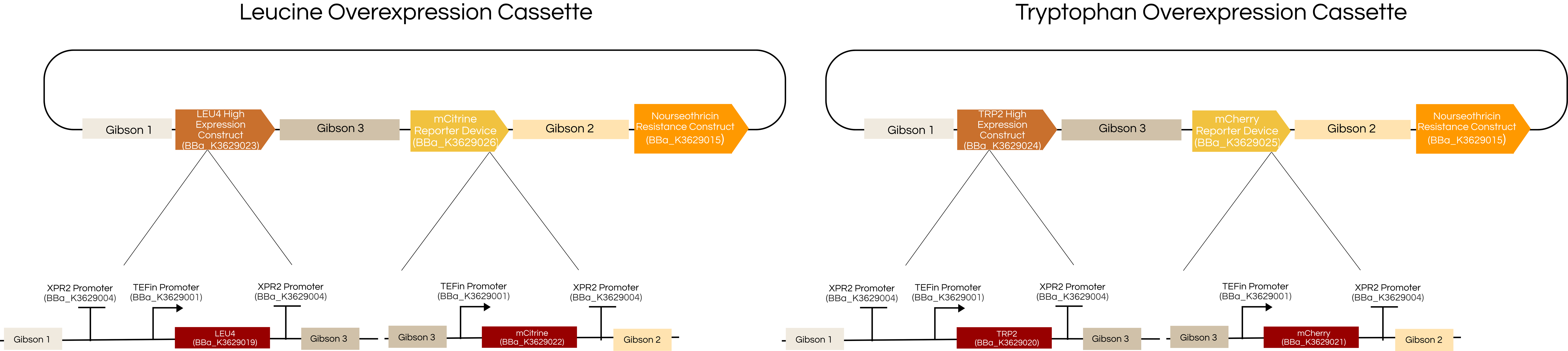
We knew in order for our cross-feeding co-culture system to work in Y. lipolytica, we needed each strain to overproduce the amino acid that the other required, similar to the S. Cerevisiae strains. Amino acid overproduction has not been implemented in Y. lipolytica before. Therefore we met with Dr. Gordon Chua, an expert in yeast functional genomics, to figure out a strategy for leucine and tryptophan overproduction in this unconventional yeast. Dr. Chua mentioned one of the easiest yet effective methods of metabolite overproduction is targeting the important regulatory genes in the biosynthesis pathway of that metabolite. Therefore, we investigated the biosynthesis pathway of leucine and tryptophan in Y. lipolytica and found that it is very similar to the pathways found in S. Cerevisiae. 2-isopropylmalate synthase (LEU4) and Anthranilate synthase component 1 (TRP2) are important regulatory enzymes involved in the biosynthesis pathway of leucine and tryptophan respectively. These enzymes prevent overproduction of the amino acids as they are feedback-inhibited by the end products of the pathway (leucine and tryptophan). Overexpression of these two enzymes could help remove the bottleneck effect of the feedback inhibition and allow for the overproduction of the amino acids. Therefore we created two high expression constructs for these two enzymes, BBa_K3629023 and BBa_K3629024. (more details on this below).
We also talked with Dr. Rodrigo Ledesma-Amaro who has years of experience working with this unconventional yeast to help determine how we can incorporate our LEU4 and TRP2 overproduction constructs into Y. lipolytica. He suggested that homologous recombination is the best method to genomic integration in this chassis. Therefore we flanked our genetic constructs with 1000bp homology arms for easy integration into Y. lipolytica genome.
Part design: Genetic Constructs
In order to implement our biocontainment strategy in Y. lipolytica , we need to engineer two strains of yeast; one that is auxotrophic for tryptophan and overproduces leucine, and one that is auxotrophic for leucine and overproduces tryptophan.
Engineering amino acid overproduction:
To engineer a leucine overproducing and a tryptophan overproducing strain we designed two overexpression cassettes that contained the following components:
Figure 1. Showing the components of the leucine overexpression cassette (left) and tryptophan overexpression cassette (right).
We designed two high expression genetic constructs; one to overexprss LEU4 BBa_K3629023, and one to overxpress TRP2 BBa_K3629024 for our overexpressing cassettes. We used the stong intronic TEF promoter BBa_K3629001 in these constructs to help ensure that LEU4 and TRP2 are expressed at high levels. TEF promoter is one of the strongest native promoters of Y. lipolytica . We included the first intron of the TEF1 gene in the sequence of the promoter since the inclusion of the first intron has shown to allow for even stronger expression.
The mCherry reporter device BBa_K3629025 and the mCitrine reporter device BBa_K3629026 were included to help distinguish and quantify the leucine and tryptophan overproducing gene in the co-culture. The leucine overproducing strain will be engineered with the mCitrine BBa_K3629026 reporter device and the tryptophan overproducing strain will be engineered with the mCherry reporter device BBa_K3629025. Since each reporter device contains a fluorescent protein that fluoresces at a distinct emission wavelength, fluorescence spectroscopy can be used to distinguish and quantify the leucine and tryptophan overproducing strains in the co-culture.
Nourseothricin resistance expression construct BBa_K3629015, optimized for expression in Y. lipolytica , was also used in our cassettes as the selection marker.
Together, the LEU4/TRP2 high expression constructs, the reporter devices (mCherry or mCitrine), and the Nourseothricin resistance expression construct made our overexpression cassette that could be used to engineer a leucine or a tryptophan overproducing Y. lipolytica . In order to allow for easy assembly of our cassettes, we made each of our genetic constructs compatible with Gibson assembly. Each part is flanked by a Gibson homology sequence that allows for all the parts to come together in a single Gibson reaction as shown in figure 1.
Engineering Auxotrophy

Figure 2. Showing the simultaneous insertion of tryptophan overexpression cassette and knocking out of LE2 gene to produce the leucine auxotroph, tryptophan overproducing strain (left) and the simultaneous insertion of leucine overexpression cassette and knocking out of TRP1 gene to produce the tryptophan auxotroph, leucine overproducing strain.
We wanted to engineer auxotrophy and amino acid overproduction in Y. lipolytica in one efficient step. Therefore, we included homology arms to upstream and downstream of the leucine and tryptophan overexpression cassettes. For example, our TRP2 overproducing cassette is flanked with homology arms to upstream and downstream of the LEU2. So that when the yeast is transformed with our cassette, through homologous recombination the TRP2 cassette would insert in the middle of the LEU2 gene, knocking out the gene. As a result, the engineered strain would become auxotrophic for leucine and overproducing for tryptophan in one engineering step as shown in figure 2.
FUTURE DIRECTIONS
What we've achieved
Though this season was certainly not what we had expected, we still achieved quite a bit considering the limited number of hours in the lab:
✔ We successfully characterised a proof of concept for our biocontainment system using a pair of already engineered S. cerevisiae strains:
- Characterised the strains in terms of auxotrophy and amino acid overproduction
- Created a viable co-culture demonstrating the ability of the strains to survive using the amino acid released by each of the complementary strains
- Demonstrated the inability of the yeast to survive in environmental conditions outside of the expected growth vessel conditions
✔ We designed and submitted a total of eight parts to the parts registry that allow for the engineering of Y. lipolytica to create complementary overproducing, auxotrophic strains.
Improving our system
Engineering multiple auxotrophies
When speaking to different contacts in academia, including Dr. Zaramberg and Dr. Mayall, we learned of ways that we could strengthen our biocontainment strategy. Currently, our yeast strains are auxotrophic for a single amino acid that is supplemented by the complementary strain. However, as an improvement to our system, we can engineer the strains to become auxotrophic for multiple amino acids rather than just one. These amino acids would then be supplemented by the second strain. Because the yeast cell requires greater supplementation to survive, it becomes more dependent on its complementary strain, reducing the chances of the cells being able to survive outside of the intended growth vessel, and thus improving the quality of our biocontainment method. As an additional future measure, different amino acids can be researched to determine which are required in the highest concentrations for a yeast’s survival. Making it deficient in one of these amino acids would also increase the effectiveness of our biocontainment method, as the yeast would have greater difficulty in surviving during the absence of this amino acid.
Improving Amino Acid Overproduction:
Our amino acid overexpression cassettes use a strong native promoter to allow for high expressions of important regulatory enzymes, LEU4 and TRP2, in biosynthetic pathways of leucine and tryptophan, respectively. This would allow the cells to overproduce the desired amino acid. An additional modification that could be made in the future to ensure even higher production of the amino acids is to overexpress mutant copies of LEU4 and TRP2 that are resistant to feedback inhibition by leucine and tryptophan respectively. LEU4 and TRP2 orthologs of Y. lipolytica are known to have similar allosteric regulatory sites to S. cerevisiae enzymes for binding of leucine and tryptophan. However, since the enzymes of Y. lipolytica are not as well characterized, the position of the regulatory allosteric site in the 3D structure of Y. lipolytica enzymes is unknown. Therefore, future analysis could be done using tools such as protein modeling to identify the position of these regulatory sites and produce mutants versions of the enzymes that are resistant to feedback inhibition and could operate at higher capacity.
REFERENCES
Balan, A., & Schenberg, A. C. G. (2005). A conditional suicide system for Saccharomyces cerevisiae relying on the intracellular production of the Serratia marcescens nuclease. Yeast, 22(3), 203-212.
Hoek, Tim A, Axelrod, Kevin, Biancalani, Tommaso, Yurtsev, Eugene A, Liu, Jinghui, & Gore, Jeff. (2016). Resource Availability Modulates the Cooperative and Competitive Nature of a Microbial Cross-Feeding Mutualism. PLoS Biology, 14(8), e1002540–e1002540. https://doi.org/10.1371/journal.pbio.1002540
Kawano, F., Suzuki, H., Furuya, A., & Sato, M. (2015). Engineered pairs of distinct photoswitches for optogenetic control of cellular proteins. Nature communications, 6(1), 1-8.
Müller, M. J., Neugeboren, B. I., Nelson, D. R., & Murray, A. W. (2014). Genetic drift opposes mutualism during spatial population expansion. Proceedings of the National Academy of Sciences of the United States of America, 111(3), 1037–1042. https://doi.org/10.1073/pnas.1313285111
Shou, W., Ram, S., & Vilar, J. M. (2007). Synthetic cooperation in engineered yeast populations. Proceedings of the National Academy of Sciences, 104(6), 1877-1882.
Torres, L., Krüger, A., Csibra, E., Gianni, E., & Pinheiro, V. B. (2016). Synthetic biology approaches to biological containment: pre-emptively tackling potential risks. Essays in biochemistry, 60(4), 393–410. https://doi.org/10.1042/EBC20160013
Xu, X., Du, Z., Liu, R., Li, T., Zhao, Y., Chen, X., & Yang, Y. (2018). A single-component optogenetic system allows stringent switch of gene expression in yeast cells. ACS synthetic biology, 7(9), 2045-2053.