OVERVIEW

Optimizing Nutrient Absorption Through Anthelmintics
To allow our target communities to maximize micronutrient and vitamin A absorption in between deworming seasons, we will engineer our yeast to produce thymol which is naturally found in thyme leaves. This will allow our yeast consumers to alleviate damage and destroy parasitic worms in the intestines. Our initial goal is to create a proof of concept in the lab. To do this, we followed the engineering design cycle to build our project:
- Understand the Problem
- Research and Ideate Solutions: Identifying Thymol
- Design Solutions: Part design
- Design Solutions: Experimental design
- Create and Test: Thymol Testing
- Evaluate
Over the next year, we will be working towards a lab proof of concept for thymol testing and production. This engineering design cycle will repeat once we have accomplished this and look towards community implementation. For more information on our plans for next year and beyond iGEM, please refer to the Future Directions section.

UNDERSTANDING THE PROBLEM
Inadequate Access to Healthcare
Mass-supplementation of Vitamin A to deficient regions have been ongoing for decades.However, even with biannual vitamin A supplementation for children and biofortified crops such as golden rice, intestinal parasites still play the villain in preventing the vitamin’s assimilation by perforating the intestines. Organizations such as the UN, WHO, and the Bill Gates Foundation couple biannual vitamin supplementation with deworming using agents like albendazole and mebendazole, but lack of clean water and proper footwear allow these parasites to return and thrive in the intestines.
Parasitic worms are among the most widespread human infections to date, affecting 2 billion people worldwide. The most common of these are Ascaris lumbricoides or the giant roundworm, hookworms, and Trichuris trichiura or the human whipworm. These worms may be obtained from direct contact with the soil, or through improper measures in hygiene. These worms cause a variety of symptoms such as stunted growth, decreased metabolic rates, physical and mental fatigue, and malnutrition (Kumar, Jain, & Jain, 2014). Since these parasites perforate the intestines, nutrition absorption is severely inhibited, causing several micronutrient supplementation programs to falter.
Albendazole has been used as an effective cure for intestinal worm infections. When treating roundworms it has a cure rate of 98.9% and egg reduction rates of 99.6%. For hookworms, it is 56.8% and 97.7% respectively, while in whipworms it is 10.% and 73.3%. However, even with these highly effective drugs the worms return and thrive until the next round of deworming because of poor hygiene due to lack of access to clean water and proper footwear.
Talking to Dr. John Gilleard and Dr. Paul E. Mains from the University of Calgary, we found out that these drugs were first intended for animals but were later implemented in humans. They believe that intestinal parasites will sooner or later develop drug resistances just as they have discovered in sheep and cattle models, which is why development of new treatments should be kept on the radar.
PART DESIGN
Thymol has been previously expressed in microbial systems such as S.cerivisiae (Aly and Abo-Sereih 2020). Since thymol is a simple monoterpenoid, its expression in Yarrowia lipolytica would not be too complex. This is because the organism is capable of producing geranyl diphosphate through the non-mevalonate (MEP) pathway, and would only need two additional genes to produce thymol. These genes are the TvTPS1 terpene synthase gene and the CYP71D178 cytochrome P450 gene from Thymus vulgaris, better known as thyme.

Gauging Thymol Production Levels
Since Dr. Gupta advised us to observe more precaution with regards to the safety and efficacy of thymol, we will test the expression of the proteins under the control of two different promoters. Our first promoter is the Translation Elongation Factor 1 (TEF1) promoter which is a well-known strong constitutive promoter in Y. lipolytica (Hong et al. 2012). Our second promoter is the Proline Dehydrogenase 2 (POX2) promoter which is known to be a relatively weaker promoter in Y. lipolytica (Shabir Hussain et al. 2017). In the future when we express thymol in Y. lipolytica, we will quantify its production and determine which is better suited to our end users’ safety.
We have not developed parts for thymol production yet, but our construct design will follow that of cellulase engineering and will be assembled through Gibson assembly, except without a signal peptide. This is because we do not need to secrete thymol as it is meant to be consumed as part of the yeast. For a more detailed description of our construct design, please click here.
For our proof-of-concept, we will be cloning and expressing this construct containing both TvTPS1 and CYP71D178 without any cellulases in Y. lipolytica. Once we have proven its functionality, we will then include these genes to our third Y. lipolytica strain containing the NpBGS using Gibson Assembly.
EXPERIMENTAL DESIGN
Thoughtful design of experiments
Below are detailed descriptions of the planned steps/experiments we will conduct to integrate the cellulase enzymes into the Y. lipolytica genome. Our first experiments will be carried out in Escherichia coli DH5α for cloning and Gibson Assembly, after which the gene cassettes will be introduced to Y. lipolytica. The experimental workflow will follow the depiction in figure 5.

To express thymol in Y. lipolytica a terpene synthase (TvTPS1) and a cytochrome P450 (CYP71D178) will be introduced. They will be assembled into the gene cassette using Gibson Assembly. The resulting Gibson plasmids will be transformed into E. coli for propagation and plasmid amplification. Following extraction, the plasmids will then be linearized with NotI, gel purified, and each will be transformed into a Y. lipolytica competent cell aliquot using the lithium acetate method to create the three strains.
If these gene cassettes are unable to be made after several attempts and troubleshooting to change the DNA concentrations/reaction conditions, then these cassettes can be assembled in many different ways, including sequentially. It is important to note that the TvTPS1 and CYP71D178 constructs are not submitted this year, but will be for iGEM 2021. Check out the appendix page and the part collection page to learn more or see the different kinds of plasmids that can be produced with our collection.
We were very fortunate to be given protocols for transformation of DNA into Y. lipolytica by Dr. Rodrigo Ledesma-Amaro. As per this protocol, cells will be made chemically competent using lithium acetate and transformed with the linearized gene cassettes containing nourseothricin as a selection marker. If the transformation is successful, we expect colonies to develop when plated on YPD with nourseothricin. If this method does not work upon changing the concentration of DNA or incubation periods, other protocols using electroporation may give higher success rates.
Colonies that appear on the nourseothricin plate will undergo colony PCR (cPCR) to verify the integration of the insert into the genome. The parts were designed to be verified using the following primers:
Forward Primer: 5’ GCTTGCTAATGTTAAGTCTCTGC 3’
Reverse Primer: 5’ GGATTCACATCAGTCAATCACC 3’
We will run the PCR products on a 1% gel electrophoresis. The virtual gel below shows the expected results of the gel.

Figure 6. Virtual gel showing the results of a Yarrowia lipolytica cPCR upon transformation with the thymol gene cassettes. The first lane contains ddH2O. The second lane contains an empty PSB1A3 vector (expected band size = 2155bp).The stand-alone strain contains TvTPS1 + CYP71D178 + Nourseothricin resistance (expected band size= 4155p), and strain 3 contains TvTPS1 + CYP71D178 +NpBGS + Nourseothricin resistance (expected band size= 8117bp). Ladder depicted is the Thermo Scientific GeneRuler 1 kb Plus DNA Ladder.
SDS PAGE gels provide a qualitative verification of protein production. We will use them to confirm that our terpene synthase and cytochrome P450 proteins are being expressed. Since both proteins will not be secreted, we will be testing our cell lysate only.
A 10% SDS PAGE will be run with the following expected results:
Table 2. Expected band sizes seen on a 10% SDS page for engineered Yarrowia lipolytica standalone strain and strain 3. The stand-alone strain contains TvTPS1 + CYP71D178 + Nourseothricin resistance. Strain 3 contains TvTPS1 + CYP71D178 +NpBGS + Nourseothricin resistance Two negative controls include Y. lipolytica transformed with only Nourseothricin resistance, and untransformed Y. lipolytica
|
Sample |
Expected Band for Cell Lysate |
|
Standalone Strain
|
Bands at:
|
|
Strain 3
|
Bands at:
|
|
Negative control: Y. lipolytica+ Nourseothricin |
Band at 20.4 kDa |
Negative control: Untransformed Y. lipolytica |
No bands corresponding to our recombinant proteins |
Gas chromatography–mass spectrometry (GC-MS) is an analytical combining the features of gas-chromatography and mass spectrometry to identify different substances and abundance within a test sample. We are using this method as it is more accurate compared to spectophotometry methods.
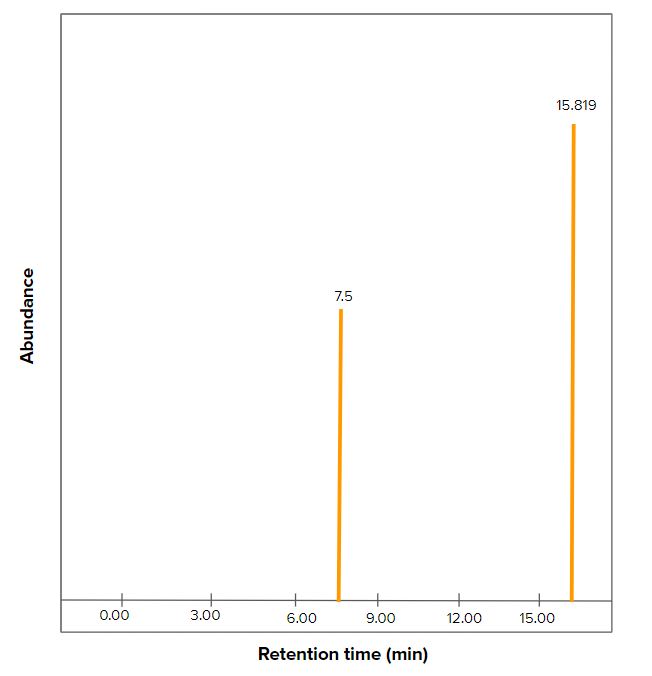
Figure 7. Expected GC/MS results of Yarrowia lipolytica upon transformation with the thymol gene cassettes. Retention time for thymol is 15.819 minutes while gamma-terpinene is 7.5 minutes. These are based on experiments by Raskovic et al. 2015 and Sigma Aldrich SLB®-IL59 analysis.
To perform this test we will be applying standard thymol powder at different concentrations to Caenorhabditis elgans and Ascaris lumbricoides and determine their growth over four days. This is because A. lumbricoides are some of the most common worms affecting our target demographic while C. elegans is used as the gold standard for anthelmintic testing.
For C. elegans, we expect a 43% mortality-rate after growing on 100nM thymol, and 100% mortality-rate in 10,000nM in Nematode Growth Media plates. This is in reference to the data collected by Marjanovic et al. in 2018, but our C. elegans strain from Dr. Mains' laboratory may produce varying results.
As for A. lumbricoides, we are uncertain of the results because thymol's effects have only been tested in the relative species A. suum in pigs and not in a petri dish. The study by Kaplan et al. in 2012 show 76.8% reduction in total worm counts of pigs administered with 1.0mg/kg thymol over four weeks.
Our next step is to co-culture the strains in the same media and measure the amount of thymol and gamma-terpinene again using GC/MS. We will also measure the presence of each strain to ensure that no strain is outcompeting the others. To do this, we can integrate a different reporter genes into each strain and measure the fluorescence at different wavelengths, providing a quantitative measure of the quantity of each strain in the co-culture. For more information on this assay check out our Bioncontainment project.
FUTURE DIRECTIONS
What we've achieved this year
✔ Collected our reagents and C. elegans for testing
✔ Extensively planned all of our experiments
✔ Identified promoter and gene sequences for thymol production in Y. lipolytica. We will be creating and codon-optimizing our constructs and have them submitted next year to contribute to the registry.
Preliminary Thymol Testing
For thymol testing, we will be working with Dr. Paul Mains who has generously offered to perform our thymol experiments on C. elegans for us. We will also get our hands on A. lubricoides worms to determine the efficacy of our drug.
Engineering Y. lipolytica
We will be performing the steps we have outlined in our experimental design. This time, we will be working with dry lab to create models of how thymol and our engineered yeast strain would affect the parasitic worms over time upon ingestion of Oviita. We will reach out again to Dr. Christopher Naugler who works with pathology informatics, and other parasitologists at the University of Calgary and beyond to optimize our system further.
References
Aly, S. E.-S., & Abo-Sereih, N. A. (2020). Application of Yeast as a Bioreactor for the Production of Microbial Flavors. International Journal of Halal Research, 2(1), 40-49. https://doi.org/10.18517/ijhr.2.1.40-49.2020
Kohlert, C., Schindler, G., März, R. W., Abel, G., Brinkhaus, B., Derendorf, H., Gräfe, E. U., & Veit, M. (2002). Systemic availability and pharmacokinetics of thymol in humans. Journal of clinical pharmacology, 42(7), 731–737. https://doi.org/10.1177/009127002401102678
Kumar, H., Jain, K., & Jain, R. (2014). A study of prevalence of intestinal worm infestation and efficacy of anthelmintic drugs. Medical Journal Armed Forces India, 70(2), 144-148. doi:10.1016/j.mjafi.2013.12.009
Kaplan RM, Storey BE, Vidyashankar AN, et al. Antiparasitic efficacy of a novel plant-based functional food using an Ascaris suum model in pigs. Acta Tropica. 2014 Nov;139:15-22. DOI: 10.1016/j.actatropica.2014.06.008.
Marjanovic, S., Bogunović, D., Milovanović, M., Marinković, D., Zdravković, N., Magaš, V., & Trailovic, M. (2018). Antihelminic Activity of Carvacrol, Thymol, Cinnamaldehyde and P-Cymen Against the Free-Living Nematode Caenorhabditis elegans and Rat Pinworm Syphacia muris. Acta Veterinaria-beograd, 68, 445-456.
Rašković, A., Pavlović, N., Kvrgić, M., Sudji, J., Mitić, G., Čapo, I., & Mikov, M. (2015). Effects of pharmaceutical formulations containing thyme on carbon tetrachloride-induced liver injury in rats. BMC complementary and alternative medicine, 15, 442. https://doi.org/10.1186/s12906-015-0966-z
Santa Cruz Biotech, “Thymol,”sc-215984.
Shabbir Hussain, M., Wheeldon, I., & Blenner, M. A. (2017). A Strong Hybrid Fatty Acid Inducible Transcriptional Sensor Built From Yarrowia lipolytica Upstream Activating and Regulatory Sequences. Biotechnology journal, 12(10), 10.1002/biot.201700248. https://doi.org/10.1002/biot.201700248
The rural school and hookworm disease : Ferrell, John Atkinson, 1880- : Free Download, Borrow, and Streaming. (1970, January 01). Retrieved October 26, 2020, from https://archive.org/details/ruralschoolhookw00ferruoft/page/11/mode/2up?q=thymol
Vercruysse J, Behnke JM, Albonico M, Ame SM, Angebault C, Bethony JM, et al. (2011) Assessment of the Anthelmintic Efficacy of Albendazole in School Children in Seven Countries Where Soil-Transmitted Helminths Are Endemic. PLoS Negl Trop Dis 5(3): e948. https://doi.org/10.1371/journal.pntd.0000948
Xie, K., Tashkin, D. P., Luo, M. Z., & Zhang, J. Y. (2019). Chronic toxicity of inhaled thymol in lungs and respiratory tracts in mouse model. Pharmacology research & perspectives, 7(5), e00516. https://doi.org/10.1002/prp2.516

